Sustainable Chitosan-Dialdehyde Cellulose Nanocrystal Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
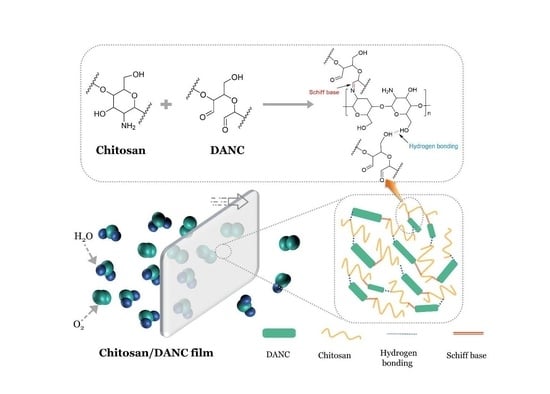
2.2. Preparation of DANC Suspension
2.3. Preparation of Chitosan/DANC Composite Films
2.4. Characterization
2.4.1. X-ray Diffraction (XRD)
2.4.2. X-ray Photoelectron Spectroscopy (XPS)
2.4.3. Transmission Electron Microscopy (TEM)
2.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.5. Scanning Electron Microscopy (SEM)
2.4.6. Optical Transmittance Measurement
2.4.7. Thermogravimetric Analysis (TGA)
2.4.8. Thickness and Mechanical Properties
2.4.9. Oxygen Permeability (OP)
2.4.10. Water Vapor Permeability (WVP)
3. Results and Discussion
3.1. Characterization of CNC and DANC
3.2. FTIR Analysis of DANC Containing Film
3.3. XPS Analysis of DANC Containing Film
3.4. Thermal Gravimetric Analysis of DANC Containing Film
3.5. Optical Properties of the DANC Containing Films
3.6. Mechanical Properties
3.7. Gas Barrier Properties
3.8. Morphology of Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Ochando-Pulido, J.M.; Victor-Ortega, M.D.; Martinez-Ferez, A. On the cleaning procedure of a hydrophilic reverse osmosis membrane fouled by secondary-treated olive mill wastewater. Chem. Eng. J. 2015, 260, 142–151. [Google Scholar] [CrossRef]
- Wang, H.; Gong, X.; Miao, Y.; Guo, X.; Liu, C.; Fan, Y.-Y.; Zhang, J.; Niu, B.; Li, W. Preparation and characterization of multilayer films composed of chitosan, sodium alginate and carboxymethyl chitosan-ZnO nanoparticles. Food Chem. 2019, 283, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Turalija, M.; Bischof, S.; Budimir, A.; Gaan, S. Antimicrobial PLA films from environment friendly additives. Compos. Part B-Eng. 2016, 102, 94–99. [Google Scholar] [CrossRef]
- Kuswandi, B. Environmental friendly food nano-packaging. Environ. Chem. Lett. 2017, 15, 205–221. [Google Scholar] [CrossRef]
- Pinem, M.P.; Wardhono, E.Y.; Nadaud, F.; Clausse, D.; Saleh, K.; Guenin, E. Nanofluid to Nanocomposite Film: Chitosan and Cellulose-Based Edible Packaging. Nanomaterials 2020, 10, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Ge, L.; Li, X.; Mu, C.; Li, D. Periodate oxidation of xanthan gum and its crosslinking effects on gelatin-based edible films. Food Hydrocoll. 2014, 39, 243–250. [Google Scholar] [CrossRef]
- Thongsomboon, W.; Serra, D.O.; Possling, A.; Hadjineophytou, C.; Hengge, R.; Cegelski, L. Phosphoethanolamine cellulose: A naturally produced chemically modified cellulose. Science 2018, 359, 334–338. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, M.; Moores, A. Review: Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem. 2016, 18, 622–637. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Jung, J.; Zhao, Y. Preparation and characterization of cellulose nanocrystals films incorporated with essential oil loaded beta-chitosan beads. Food Hydrocoll. 2017, 69, 164–172. [Google Scholar] [CrossRef]
- Soni, B.; Hassan, E.B.; Schilling, M.W.; Mahmoud, B. Transparent bionanocomposite films based on chitosan and TEMPO-oxidized cellulose nanofibers with enhanced mechanical and barrier properties. Carbohydr. Polym. 2016, 151, 779–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, S.C.; da Silva, A.A.; Costa, D.B.; Costa, C.M.; Lanceros-Mendez, S.; Tamano Maciavello, M.N.; Gomez Ribelles, J.L.; Sentanin, F.; Pawlicka, A.; Silva, M.M. Thermal-mechanical behaviour of chitosan-cellulose derivative thermoreversible hydrogel films. Cellulose 2015, 22, 1911–1929. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Blanco, A.; Negro, C. Study of The Reaction Mechanism to Produce Nanocellulose-Graft-Chitosan Polymer. Nanomaterials 2018, 8, 883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Lv, M.; Anderson, D.P.; Chang, P.R. Natural polysaccharide composites based on modified cellulose spheres and plasticized chitosan matrix. Food Hydrocoll. 2017, 66, 276–285. [Google Scholar] [CrossRef]
- Khalil, H.; Saurabh, C.K.; Adnan, A.S.; Fazita, M.R.N.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; Haafiz, M.K.M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar] [CrossRef]
- Wang, J.W.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z.Y. Moisture and Oxygen Barrier Properties of Cellulose Nanomaterial-Based Films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Hanif, Z.; Park, S.A.; Choi, B.G.; Tran, T.H.; Hwang, D.S.; Park, J.; Hwang, S.Y.; Oh, D.X. Sustainable Boron Nitride Nanosheet-Reinforced Cellulose Nanofiber Composite Film with Oxygen Barrier without the Cost of Color and Cytotoxicity. Polymers 2018, 10, 501. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Jeon, H.; Jegal, J.; Kim, J.H.; Yang, H.C.; Park, J.; Oh, D.X.; Hwang, S.Y. Trans crystallization behavior and strong reinforcement effect of cellulose nanocrystals on reinforced poly(butylene succinate) nanocomposites. RSC Adv. 2018, 8, 15389–15398. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.; Chiu, F.-C. Cellulose nanocrystals reinforced kappa-carrageenan based UV resistant transparent bionanocomposite films for sustainable packaging applications. Carbohydr. Polym. 2019, 211, 181–194. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Kassab, Z.; Aziz, F.; Hannache, H.; Ben Youcef, H.; El Achaby, M. Improved mechanical properties of k-carrageenan-based nanocomposite films reinforced with cellulose nanocrystals. Int. J. Biol. Macromol. 2019, 123, 1248–1256. [Google Scholar] [CrossRef]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Kumar, K.Y. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydr. Polym. 2016, 153, 600–618. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Mattoso, L.H.C.; Avena-Bustillos, R.J.; Ceotto Filho, G.; Munford, M.L.; Wood, D.; McHugh, T.H. Nanocellulose Reinforced Chitosan Composite Films as Affected by Nanofiller Loading and Plasticizer Content. J. Food Sci. 2010, 75, N1–N7. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yan, D.; Lu, Q.; Jiang, X. Cationic surface modification of nanocrystalline cellulose as reinforcements for preparation of the chitosan-based nanocomposite films. Cellulose 2017, 24, 163–174. [Google Scholar] [CrossRef]
- Ferrer, A.; Filpponen, I.; Rodríguez, A.; Laine, J.; Rojas, O.J. Valorization of residual Empty Palm Fruit Bunch Fibers (EPFBF) by microfluidization: Production of nanofibrillated cellulose and EPFBF nanopaper. Bioresour. Technol. 2012, 125, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Vu, K.D.; Chauve, G.; Bouchard, J.; Riedl, B.; Lacroix, M. Optimization of microfluidization for the homogeneous distribution of cellulose nanocrystals (CNCs) in biopolymeric matrix. Cellulose 2014, 21, 3457–3468. [Google Scholar] [CrossRef] [Green Version]
- Liimatainen, H.; Visanko, M.; Sirvio, J.A.; Hormi, O.E.O.; Niinimaki, J. Enhancement of the Nanofibrillation of Wood Cellulose through Sequential Periodate-Chlorite Oxidation. Biomacromolecules 2012, 13, 1592–1597. [Google Scholar] [CrossRef]
- Sirviö, J.; Honka, A.; Liimatainen, H.; Niinimäki, J.; Hormi, O. Synthesis of highly cationic water-soluble cellulose derivative and its potential as novel biopolymeric flocculation agent. Carbohydr. Polym. 2011, 86, 266–270. [Google Scholar] [CrossRef]
- Sirvio, J.A.; Kolehmainen, A.; Visanko, M.; Liimatainen, H.; Niinimaki, J.; Hormi, O.E.O. Strong, Self-Standing Oxygen Barrier Films from Nanocelluloses Modified with Regioselective Oxidative Treatments. ACS Appl. Mater. Interfaces 2014, 6, 14384–14390. [Google Scholar] [CrossRef]
- Plappert, S.F.; Quraishi, S.; Pircher, N.; Mikkonen, K.S.; Veigel, S.; Klinger, K.M.; Potthast, A.; Rosenau, T.; Liebner, F.W. Transparent, Flexible, and Strong 2,3-Dialdehyde Cellulose Films with High Oxygen Barrier Properties. Biomacromolecules 2018, 19, 2969–2978. [Google Scholar] [CrossRef]
- Yao, M.; Wang, Z.; Liu, Y.; Yang, G.; Chen, J. Preparation of dialdehyde cellulose graftead graphene oxide composite and its adsorption behavior for heavy metals from aqueous solution. Carbohydr. Polym. 2019, 212, 345–351. [Google Scholar] [CrossRef]
- Xu, Q.; Ji, Y.; Sun, Q.; Fu, Y.; Xu, Y.; Jin, L. Fabrication of Cellulose Nanocrystal/Chitosan Hydrogel for Controlled Drug Release. Nanomaterials 2019, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Kang, L.; Wang, B.; Chen, K.F.; Tian, X.J.; Ge, Z.; Zeng, J.S.; Xu, J.; Gao, W.H. Controlled Release and Long-Term Antibacterial Activity of Dialdehyde Nanofibrillated Cellulose/Silver Nanoparticle Composites. ACS Sustain. Chem. Eng. 2019, 7, 1146–1158. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Q.; Zhang, X.; Li, Y.; Li, B.; Liu, S. Cellulose-based peptidopolysaccharides as cationic antimicrobial package films. Int. J. Biol. Macromol. 2019, 128, 673–680. [Google Scholar] [CrossRef]
- George, D.; Maheswari, P.U.; Begum, K.M.M.S. Synergic formulation of onion peel quercetin loaded chitosan-cellulose hydrogel with green zinc oxide nanoparticles towards controlled release, biocompatibility, antimicrobial and anticancer activity. Int. J. Biol. Macromol. 2019, 132, 784–794. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Liimatainen, H.; Suopajarvi, T.; Sirvio, J.; Hormi, O.; Niinimaki, J. Fabrication of cationic cellulosic nanofibrils through aqueous quaternization pretreatment and their use in colloid aggregation. Carbohydr. Polym. 2014, 103, 187–192. [Google Scholar] [CrossRef]
- Lu, T.; Li, Q.; Chen, W.; Yu, H. Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos. Sci. Technol. 2014, 94, 132–138. [Google Scholar] [CrossRef]
- Li, H.; Wu, B.; Mu, C.; Lin, W. Concomitant degradation in periodate oxidation of carboxymethyl cellulose. Carbohydr. Polym. 2011, 84, 881–886. [Google Scholar] [CrossRef]
- Okita, Y.; Saito, T.; Isogai, A. Entire Surface Oxidation of Various Cellulose Microfibrils by TEMPO-Mediated Oxidation. Biomacromolecules 2010, 11, 1696–1700. [Google Scholar] [CrossRef]
- Rattaz, A.; Mishra, S.P.; Chabot, B.; Daneault, C. Cellulose nanofibres by sonocatalysed-TEMPO-oxidation. Cellulose 2011, 18, 585. [Google Scholar] [CrossRef]
- Segal, L.C.; Creely, J.; Martin, A.E.J.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Krochta, J.M.; Baldwin, E.A.; Nisperos-Carriedo, M.O. Edible Coatings and Films to Improve Food Quality; Technomic Publ. Co.: Lancaster, PA, USA, 1994. [Google Scholar]
- Noshirvani, N.; Ghanbarzadeh, B.; Gardrat, C.; Rezaei, M.R.; Hashemi, M.; Le Coz, C.; Coma, V. Cinnamon and ginger essential oils to improve antifungal, physical and mechanical properties of chitosan-carboxymethyl cellulose films. Food Hydrocoll. 2017, 70, 36–45. [Google Scholar] [CrossRef]
- Chang, W.S.; Chen, H.H. Physical properties of bacterial cellulose composites for wound dressings. Food Hydrocoll. 2016, 53, 75–83. [Google Scholar] [CrossRef]
- Woggum, T.; Sirivongpaisal, P.; Wittaya, T. Properties and characteristics of dual-modified rice starch based biodegradable films. Int. J. Biol. Macromol. 2014, 67, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Hirota, M.; Tamura, N.; Kimura, S.; Fukuzumi, H.; Heux, L.; Isogai, A. Individualization of Nano-Sized Plant Cellulose Fibrils by Direct Surface Carboxylation Using TEMPO Catalyst under Neutral Conditions. Biomacromolecules 2009, 10, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nishiyama, Y.; Putaux, J.-L.; Vignon, M.; Isogai, A. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef]
- Luo, H.Z.; Lan, H.; Cha, R.T.; Yu, X.N.; Gao, P.Y.; Zhang, P.; Zhang, C.L.; Han, L.; Jiang, X.Y. Dialdehyde Nanocrystalline Cellulose as Antibiotic Substitutes against Multidrug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2021, 13, 33802–33811. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Johnson, G.P.; French, A.D.; Forsyth, V.T.; Langan, P. Neutron Crystallography, Molecular Dynamics, and Quantum Mechanics Studies of the Nature of Hydrogen Bonding in Cellulose I-beta. Biomacromolecules 2008, 9, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.J.; Chavan, V.B. A Study of Crystallinity Changes in Oxidized Celluloses. Polym. Degrad. Stabil. 1995, 49, 245–250. [Google Scholar] [CrossRef]
- Kim, U.J.; Kuga, S.; Wada, M.; Okano, T.; Kondo, T. Periodate oxidation of crystalline cellulose. Biomacromolecules 2000, 1, 488–492. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, P.; Li, Y.; He, X.; Dai, Y.; Tan, Z. Preparation and antibacterial activity of a cellulose-based Schiff base derived from dialdehyde cellulose and L-lysine. Ind. Crop. Prod. 2020, 145, 112126. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, J.; Zhang, L. Structure and Properties of the Nanocomposite Films of Chitosan Reinforced with Cellulose Whiskers. J. Polym. Sci. Part B-Polym. Phys. 2009, 47, 1069–1077. [Google Scholar] [CrossRef]
- Hassan, E.A.; Hassan, M.L.; Abou-Zeid, R.E.; El-Wakil, N.A. Novel nanofibrillated cellulose/chitosan nanoparticles nanocomposites films and their use for paper coating. Ind. Crop. Prod. 2016, 93, 219–226. [Google Scholar] [CrossRef]
- Ridolfi, D.M.; Lemes, A.P.; de Oliveira, S.; Justo, G.Z.; Palladino, M.V.; Duran, N. Electrospun poly(ethylene oxide)/chitosan nanofibers with cellulose nanocrystals as support for cell culture of 3T3 fibroblasts. Cellulose 2017, 24, 3353–3365. [Google Scholar] [CrossRef]
- He, J.-X.; Tan, W.-L.; Han, Q.-M.; Cui, S.-Z.; Shao, W.; Sang, F. Fabrication of silk fibroin/cellulose whiskers-chitosan composite porous scaffolds by layer-by-layer assembly for application in bone tissue engineering. J. Mater. Sci. 2016, 51, 4399–4410. [Google Scholar] [CrossRef]
- Ostadhossein, F.; Mahmoudi, N.; Morales-Cid, G.; Tamjid, E.; Javier Navas-Martos, F.; Soriano-Cuadrado, B.; Lopez Paniza, J.M.; Simchi, A. Development of Chitosan/Bacterial Cellulose Composite Films Containing Nanodiamonds as a Potential Flexible Platform for Wound Dressing. Materials 2015, 8, 6401–6418. [Google Scholar] [CrossRef]
- Tran, C.D.; Mututuvari, T.M. Cellulose, Chitosan, and Keratin Composite Materials. Controlled Drug Release. Langmuir 2015, 31, 1516–1526. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Nie, S.; Zhu, H.; Wang, S.; Song, X.; Qin, C. Extraction of hemicellulose by hot water to reduce adsorbable organic halogen formation in chlorine dioxide bleaching of bagasse pulp. Ind. Crop. Prod. 2017, 96, 178–185. [Google Scholar] [CrossRef]
- Kim, J.; Cai, Z.; Lee, H.S.; Choi, G.S.; Lee, D.H.; Jo, C. Preparation and characterization of a Bacterial cellulose/Chitosan composite for potential biomedical application. J. Polym. Res. 2011, 18, 739–744. [Google Scholar] [CrossRef]
- Yang, X.; Niu, X.; Mo, Z.; Guo, R.; Liu, N.; Zhao, P.; Liu, Z. Perylene-functionalized graphene sheets modified with chitosan for voltammetric discrimination of tryptophan enantiomers. Microchim. Acta 2019, 186, 333. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.F.B.; Pinto, R.J.B.; Vaz Serra, V.I.R.C.; Silvestre, A.J.D.; Trindade, T.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Daina, S.; Sadocco, P.; Freire, C.S.R. Fluorescent Bioactive Corrole Grafted-Chitosan Films. Biomacromolecules 2016, 17, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, R.; Liu, R.; Du, X.; Meng, R.; Liu, L.; Yao, J. Rapid capture and visual detection of copper ions in aqueous solutions and biofluids using a novel cellulose-Schiff base. Cellulose 2018, 25, 6947–6961. [Google Scholar] [CrossRef]
- Chen, C.; Sun, W.; Lv, H.; Li, H.; Wang, Y.; Wang, P. Spacer arm-facilitated tethering of laccase on magnetic polydopamine nanoparticles for efficient biocatalytic water treatment. Chem. Eng. J. 2018, 350, 949–959. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, X.; Gu, H.; Li, F.; Huang, W.; Liang, L.; Ye, Z. Highly selective colorimetric sensing of Cu2+ using a Schiff base derivative immobilized on polyvinyl alcohol microspheres. New J. Chem. 2018, 42, 11682–11688. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Ou, J.; Chen, L.; Ye, M. Construction of hierarchically porous monoliths from covalent organic frameworks (COFs) and their application for bisphenol A removal. J. Hazard. Mater. 2018, 355, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, R.A.; Salmieri, S.; Le Tien, C.; Riedl, B.; Bouchard, J.; Chauve, G.; Tan, V.; Kamal, M.R.; Lacroix, M. Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan based nanocomposite films. Carbohydr. Polym. 2012, 90, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Baran, T.; Sargin, I.; Kaya, M.; Mentes, A. Green heterogeneous Pd(II) catalyst produced from chitosan-cellulose micro beads for green synthesis of biaryls. Carbohydr. Polym. 2016, 152, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Huq, T.; Salmieri, S.; Khan, A.; Khan, R.A.; Le, T.C.; Riedl, B.; Fraschini, C.; Bouchard, J.; Uribecalderon, J.; Kamal, M.R. Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film. Carbohydr. Polym. 2012, 90, 1757–1763. [Google Scholar] [CrossRef]
- Mehrali, M.; Shirazi, F.S.; Mehrali, M.; Metselaar, H.S.; Kadri, N.A.; Osman, N.A. Dental implants from functionally graded materials. J. Biomed. Mater. Res. Part A 2013, 101, 3046. [Google Scholar] [CrossRef]
- Cheng, S.L.; Zhang, Y.P.; Cha, R.T.; Yang, J.L.; Jiang, X.Y. Water-soluble nanocrystalline cellulose films with highly transparent and oxygen barrier properties. Nanoscale 2016, 8, 973–978. [Google Scholar] [CrossRef]
- Toivonen, M.S.; Kurki-Suonio, S.; Schacher, F.H.; Hietala, S.; Rojas, O.J.; Ikkala, O. Water-Resistant, Transparent Hybrid Nanopaper by Physical Cross-Linking with Chitosan. Biomacromolecules 2015, 16, 1062–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Pascoal Neto, C.; Gandini, A.; Berglund, L.A.; Salmén, L. Transparent chitosan films reinforced with a high content of nanofibrillated cellulose. Carbohydr. Polym. 2010, 81, 394–401. [Google Scholar] [CrossRef]
- Wu, T.; Farnood, R.; O’Kelly, K.; Chen, B. Mechanical behavior of transparent nanofibrillar cellulose–chitosan nanocomposite films in dry and wet conditions. J. Mech. Behav. Biomed. Mater. 2014, 32, 279–286. [Google Scholar] [CrossRef]
- Aulin, C.; Karabulut, E.; Tran, A.; Wagberg, L.; Lindstrom, T. Transparent Nanocellulosic Multilayer Thin Films on Polylactic Acid with Tunable Gas Barrier Properties. ACS Appl. Mater. Interfaces 2013, 5, 7352–7359. [Google Scholar] [CrossRef]
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Nair, P. Physical and chemical reinforcement of chitosan film using nanocrystalline cellulose and tannic acid. Cellulose 2015, 22, 2529–2541. [Google Scholar] [CrossRef]
- de Mesquita, J.P.; Donnici, C.L.; Pereira, F.V. Biobased Nanocomposites from Layer-by-Layer Assembly of Cellulose Nanowhiskers with Chitosan. Biomacromolecules 2010, 11, 473–480. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulose Whiskers versus Microfibrils: Influence of the Nature of the Nanoparticle and its Surface Functionalization on the Thermal and Mechanical Properties of Nanocomposites. Biomacromolecules 2009, 10, 425–432. [Google Scholar] [CrossRef]
- Colom, X.; Carrasco, F.; Pagès, P.; Cañavate, J. Effects of different treatments on the interface of HDPE/lignocellulosic fiber composites. Compos. Sci. Technol. 2003, 63, 161–169. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Iannoni, A.; Saino, E.; Visai, L.; Berglund, L.A.; Kenny, J.M. Multifunctional bionanocomposite films of poly(lactic acid), cellulose nanocrystals and silver nanoparticles. Carbohydr. Polym. 2012, 87, 1596–1605. [Google Scholar] [CrossRef]
- Cao, Z.; Shen, Z.; Luo, X.; Zhang, H.; Liu, Y.; Cai, N.; Xue, Y.; Yu, F. Citrate-modified maghemite enhanced binding of chitosan coating on cellulose porous membranes for potential application as wound dressing. Carbohydr. Polym. 2017, 166, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Quilaqueo Gutiérrez, M.; Echeverría, I.; Ihl, M.; Bifani, V.; Mauri, A.N. Carboxymethylcellulose–montmorillonite nanocomposite films activated with murta (Ugni molinae Turcz) leaves extract. Carbohydr. Polym. 2012, 87, 1495–1502. [Google Scholar] [CrossRef]
- Cao, Z.; Luo, X.; Zhang, H.; Fu, Z.; Shen, Z.; Cai, N.; Xue, Y.; Yu, F. A facile and green strategy for the preparation of porous chitosan-coated cellulose composite membranes for potential applications as wound dressing. Cellulose 2016, 23, 1349–1361. [Google Scholar] [CrossRef]
- Hashemi Doulabi, A.; Mirzadeh, H.; Imani, M.; Samadi, N. Chitosan/polyethylene glycol fumarate blend film: Physical and antibacterial properties. Carbohydr. Polym. 2013, 92, 48–56. [Google Scholar] [CrossRef]
- Fabra, M.J.; Hambleton, A.; Talens, P.; Debeaufort, F.; Chiralt, A. Effect of ferulic acid and α-tocopherol antioxidants on properties of sodium caseinate edible films. Food Hydrocoll. 2011, 25, 1441–1447. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Lopez, J.; Kenny, J.M. Bionanocomposite films based on plasticized PLA-PHB/cellulose nanocrystal blends. Carbohydr. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Ben Dhieb, F.; Dil, E.J.; Tabatabaei, S.H.; Mighri, F.; Ajji, A. Effect of nanoclay orientation on oxygen barrier properties of LbL nanocomposite coated films. RSC Adv. 2019, 9, 1632–1641. [Google Scholar] [CrossRef] [Green Version]
- Aulin, C.; Salazar-Alvarez, G.; Lindström, T. High strength, flexible and transparent nanofibrillated cellulose-nanoclay biohybrid films with tunable oxygen and water vapor permeability. Nanoscale 2012, 4, 6622–6628. [Google Scholar] [CrossRef]
- Abdellatief, A.; Welt, B.A. Comparison of New Dynamic Accumulation Method for Measuring Oxygen Transmission Rate of Packaging against the Steady-State Method Described by ASTM D3985. Packag. Technol. Sci. 2013, 26, 281–288. [Google Scholar] [CrossRef]
- Samir, M.; Alloin, F.; Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef]










| Sample | CNC | DANC |
|---|---|---|
| Aldehyde content, mmol/g | – | 8.78 |
| Carboxylate content, mmol/g | 0.0065 | 0.014 |
| CrI, % | 94.40 | 86.02 |
| yield, % | 67.2 | 79.2 |
| Sample | TS, MPa | Eb, % | WVP, g·mm·m−2·atm−1·day−1 | WVRT, g·m−2·day−1 | OP, cm3·mm·m−2·day−1·atm−1 | ORT, cm3·m−2·day−1 |
|---|---|---|---|---|---|---|
| 75/25 | 14.2 | 3.05 | 47.64 | 1476.84 | 0.11 | 3.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Wang, S.; Liu, B.; Yao, S.; Dai, Y.; Zhou, L.; Qin, C.; Fatehi, P. Sustainable Chitosan-Dialdehyde Cellulose Nanocrystal Film. Materials 2021, 14, 5851. https://doi.org/10.3390/ma14195851
Gao C, Wang S, Liu B, Yao S, Dai Y, Zhou L, Qin C, Fatehi P. Sustainable Chitosan-Dialdehyde Cellulose Nanocrystal Film. Materials. 2021; 14(19):5851. https://doi.org/10.3390/ma14195851
Chicago/Turabian StyleGao, Cong, Shuo Wang, Baojie Liu, Shuangquan Yao, Yi Dai, Long Zhou, Chengrong Qin, and Pedram Fatehi. 2021. "Sustainable Chitosan-Dialdehyde Cellulose Nanocrystal Film" Materials 14, no. 19: 5851. https://doi.org/10.3390/ma14195851