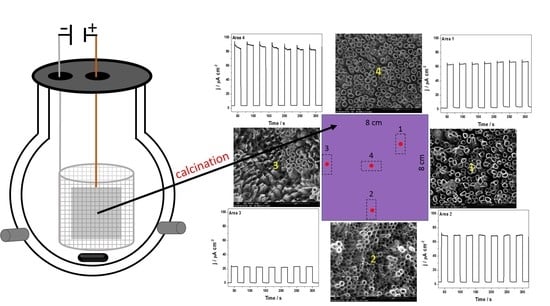
Scaling Up the Process of Titanium Dioxide Nanotube Synthesis and Its Effect on Photoelectrochemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of TiO2 Nanotubes
2.2. Characterization Techniques
2.2.1. Morphology and Crystal Structure
2.2.2. Photoelectrochemical Measurements
3. Results and Discussion
3.1. Morphology
3.2. UV–Vis and Raman Spectroscopy
3.3. Photoelectrochemical Properties
3.4. The Solar to Hydrogen Conversion Efficiency
3.5. Flat Band Potential and Carrier Density
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bessegato, G.G.; Guaraldo, T.T.; De Brito, J.F. Achievements and Trends in Photoelectrocatalysis: From Environmental to Energy Applications. Electrocatal 2015, 6, 415–441. [Google Scholar] [CrossRef] [Green Version]
- Kandiel, T.A.; Feldhoff, A.; Robben, L.; Dillert, R.; Bahnemann, D.W. Tailored Titanium Dioxide Nanomaterials: Anatase Nanoparticles and Brookite Nanorods as Highly Active Photocatalysts. Chem. Mater. 2010, 22, 2050–2060. [Google Scholar] [CrossRef]
- Mena, E.; Martín de Vidales, M.J.; Mesones, S.; Marugán, J. Influence of anodization mode on the morphology and photocatalytic activity of TiO2-NTs array large size electrodes. Catal. Today 2018, 313, 33–39. [Google Scholar] [CrossRef]
- Sopha, H.; Baudys, M.; Krbal, M.; Zazpe, R.; Prikryl, J.; Krysa, J.; Macak, J.M. Scaling up anodic TiO2 nanotube layers for gas phase photocatalysis. Electrochem. Commun. 2018, 97, 91–95. [Google Scholar] [CrossRef]
- Macak, J.M.; Zlamal, M.; Krysa, J. Self-Organized TiO2 Nanotube Layers as Highly Efficient Photocatalysts. Small 2007, 6, 300–304. [Google Scholar] [CrossRef]
- Peng, B.X.; Chen, A. Large-Scale Synthesis and Characterization of TiO2 -Based Nanostructures on Ti Substrates. Adv. Funct. Mater. 2006, 16, 1355–1362. [Google Scholar] [CrossRef]
- Kalbacova, K.; Macak, J.M.; Mierke, C.T.; Schmuki, P. TiO2 nanotubes: Photocatalyst for cancer cell killing. Phys. Status Solidi 2008, 196, 194–196. [Google Scholar] [CrossRef]
- Paramasivam, I.; Jha, H.; Liu, N.; Schmuki, P. A Review of Photocatalysis using Self-organized TiO2 Nanotubes and Other Ordered Oxide Nanostructures. Small 2012, 20, 3073–3103. [Google Scholar] [CrossRef]
- Paramasivam, I.; Nah, Y.; Das, C.; Shrestha, N.K.; Schmuk, P. WO3/TiO2 Nanotubes with Strongly Enhanced Photocatalytic Activity Related papers. Chem. Eur. J. 2010, 16, 8993–8997. [Google Scholar] [CrossRef] [PubMed]
- Beranek, R.; Macak, J.M.; Gärtner, M.; Meyer, K.; Schmuki, P. Enhanced visible light photocurrent generation at surface-modified TiO2 nanotubes. Electrochim. Acta 2009, 54, 2640–2646. [Google Scholar] [CrossRef]
- Trzciński, K.; Szkoda, M.; Siuzdak, K.; Sawczak, M.; Lisowska-Oleksiak, A. Electrochemical and photoelectrochemical characterization of photoanodes based on titania nanotubes modified by a BiVO4 thin film and gold nanoparticles. Electrochim. Acta 2016, 222, 421–428. [Google Scholar] [CrossRef]
- Roy, P.; Kim, D.; Lee, K.; Schmuki, P. TiO2 nanotubes and their application in dye-sensitized solar cells. Nanoscale 2010, 2, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of Highly-Ordered TiO2 Nanotube Arrays in Dye-Sensitized Solar Cells. Nano Lett. 2006, 2, 215–218. [Google Scholar] [CrossRef]
- Arrays, N.; Chen, P.; Takata, M.; Uchida, S.; Miura, H.; Sumioka, K.; Zakeeruddin, S.M.; Gra, M. Application of Highly Ordered TiO2 Sensitized Solar Cells. ACS Nano 2008, 2, 1113–1116. [Google Scholar]
- Ghicov, A.; Albu, S.P.; Macak, J.M.; Schmuk, P. High-Contrast Electrochromic Switching UsingTransparent Lift-Off Layers of Self-Organized TiO2 Nanotube. Small 2008, 4, 1063–1066. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, D.; Schmuki, P. Electrochromic properties of anodically grown mixed V2O5–TiO2 nanotubes. Electrochem. Commun. 2011, 13, 1021–1025. [Google Scholar] [CrossRef]
- Siuzdak, K.; Szkoda, M.; Sawczak, M.; Karczewski, J.; Ryl, J.; Cenian, A. Ordered titania nanotubes layer selectively annealed by laser beam for high contrast electrochromic switching. Thin Solid Films 2018, 659, 48–56. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 Nanotubes: Recent Advances in Synthesis and Gas Sensing Properties. Sensors 2013, 13, 14813–14838. [Google Scholar] [CrossRef]
- Li, X.; Yao, J.; Liu, F.; He, H.; Zhou, M.; Mao, N.; Xiao, P.; Zhang, Y. Nickel/Copper nanoparticles modified TiO2 nanotubes for non-enzymatic glucose biosensors. Sens. Actuators B Chem. 2013, 181, 501–508. [Google Scholar] [CrossRef]
- Grochowska, K.; Siuzdak, K.; Sokołowski, M.; Karczewski, J. Properties of ordered titanium templates covered with Au thin films for SERS applications. Appl. Surf. Sci. 2016, 388, 716–722. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Hubler, R.; Shinkai, R.S.A.; Teixeira, E.R. Application of TiO2 Nanotubes as a Drug Delivery System for Biomedical Implants: A Critical Overview. ChemistrySelect 2018, 3, 11180–11189. [Google Scholar] [CrossRef]
- Junkar, I.; Kulkarni, M.; Dra, B.; Mazare, A.; Fla, A.; Drobne, D.; Humpolí, P.; Resnik, M.; Schmuki, P.; Mozeti, M. Bioelectrochemistry Influence of various sterilization procedures on TiO2 nanotubes used for biomedical devices. Bioelectrochemistry 2016, 109, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Vega, V.; Vázquez, M.; Prida, V.M.; Hernandez-Velez, M.; Manova, E.; Aranda, P.; Ruiz-Hitzky, E. Influence of Anodic Conditions on Self-ordered Growth of Highly Aligned Titanium Oxide Nanopores. Nanoscale Res. Lett. 2007, 2, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Macak, J.M.; Sirotna, K.; Schmuki, P. Self-organized porous titanium oxide prepared in Na2SO4/NaF electrolytes. Electrochim. Acta 2005, 50, 3679–3684. [Google Scholar] [CrossRef]
- Albu, B.S.P.; Ghicov, A.; Aldabergenova, S.; Drechsel, P.; Leclere, D.; Thompson, G.E.; Macak, J.M.; Schmuki, P. Formation of Double-Walled TiO2 Nanotubes and Robust Anatase Membranes. Adv. Mater. 2008, 20, 4135–4139. [Google Scholar]
- Bauer, S.; Kleber, S.; Schmuki, P. TiO2 nanotubes: Tailoring the geometry in H3PO4/HF electrolytes. Electrochem. Commun. 2006, 8, 1321–1325. [Google Scholar] [CrossRef]
- Vega, V.; Montero-moreno, J.M.; García, J.; Prida, V.M.; Rahimi, W.; Waleczek, M. Long-Range Hexagonal Arrangement of TiO2 Nanotubes by Soft Lithography-Guided Anodization. Electrochim. Acta 2016, 203, 51–58. [Google Scholar] [CrossRef]
- Szkoda, M.; Trzciński, K.; Lisowska-Oleksiak, A.; Siuzdak, K. Electrochemical and photoelectrochemical properties of the interface between titania nanotubes covered by conducting polymer in aqueous by conducting polymer in aqueous electrolytes—The effect of various geometry and electrolytes concentration. Appl. Surf. Sci. 2018, 448, 309–319. [Google Scholar] [CrossRef]
- Xiang, C.; Sun, L.; Wang, Y.; Wang, G.; Zhao, X.; Zhang, S. Large-Scale, Uniform, and Superhydrophobic Titania Nanotubes at the Inner Surface of 1000 mm Long Titanium Tubes. J. Phys. Chem. C 2017, 121, 15448–15455. [Google Scholar] [CrossRef]
- Szkoda, M.; Lisowska-Oleksiak, A.; Grochowska, K.; Skowroński, Ł.; Karczewski, J.; Siuzdak, K. Semi-transparent ordered TiO2 nanostructures prepared by anodization of titanium thin films deposited onto the FTO substrate. Appl. Surf. Sci. 2016, 381, 36–41. [Google Scholar] [CrossRef]
- Pu, P.; Cachet, H.; Ngaboyamahina, E.; Sutter, E.M.M. Relation between morphology and conductivity in TiO2 nanotube arrays: An electrochemical impedance spectrometric investigation. J. Solid State Electrochem. 2013, 17, 817–828. [Google Scholar] [CrossRef]
- Stagi, L.; Carbonaro, C.M.; Corpino, R.; Chiriu, D.; Ricci, P.C. Light induced TiO2 phase transformation: Correlation with luminescent surface defects. Phys. Status Solidi B 2015, 129, 124–129. [Google Scholar] [CrossRef]
- Lai, Y.K.; Huang, J.Y.; Zhang, H.F.; Subramaniam, V.P.; Tang, Y.X.; Gong, D.G.; Sundar, L.; Sun, L.; Chen, Z.; Lin, C.J. Nitrogen-doped TiO2 nanotube array films with enhanced photocatalytic activity under various light sources. J. Hazard. Mater. 2010, 184, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, N.; Yang, H.; Leng, X.; Zou, J. Interface feature characterization and Schottky interfacial layer confirmation of TiO2 nanotube array film. Appl. Surf. Sci. 2015, 335, 849–860. [Google Scholar] [CrossRef]
- Wang, L.; Han, J.; Zhu, R.; Jaye, C.; Liu, H.; Li, Z.; Taylor, G.T.; Fischer, D.A.; Appenzeller, J.; Wong, G.G. Probing the Dependence of Electron Transfer on Size and Coverage in Carbon Nanotube–Quantum Dot Heterostructures. J. Phys. Chem. C 2015, 119, 26327–26338. [Google Scholar] [CrossRef]
- Kongkanand, A.; Tvrdy, K.; Takechi, K.; Kuno, M.; Kamat, P.V.; Dame, N. Quantum Dot Solar Cells. Tuning Photoresponse through Size and Shape Control of CdSe–TiO2 Architecture. J. Am. Chem. Soc. 2008, 130, 4007–4015. [Google Scholar] [CrossRef]
- Sharma, D.; Upadhyay, S.; Verma, A.; Satsangi, V.R.; Shrivastav, R.; Dass, S. Nanostructured Ti-Fe2O3/CuO heterojunction photoelectrode for ef fi cient hydrogen production. Thin Solid Films 2015, 574, 125–131. [Google Scholar] [CrossRef]








| No. | Sample | F (nm) | D (nm) | H (µm) | Sr (cm2) |
|---|---|---|---|---|---|
| 1 | NT4 | 22 | 140 | 2.9 | 80.8 |
| 2 | NT16 | 25 | 170 | 2.2 | 50.3 |
| 3 | NT25 | 26 | 180 | 2.1 | 50.2 |
| 4 | NT36 | 28 | 200 | 1.9 | 36.8 |
| 5 | NT64 | 26 | 150 | 1.8 | 67.1 |
| Electrode | Area | J (µA·cm−2) | η (%) |
|---|---|---|---|
| NT4 | 1 | 83 | 0.051 |
| 2 | 81 | 0.050 | |
| NT16 | 1 | 80 | 0.049 |
| 2 | 82 | 0.050 | |
| 3 | 99 | 0.055 | |
| NT25 | 1 | 66 | 0.044 |
| 2 | 79 | 0.052 | |
| 3 | 82 | 0.055 | |
| NT36 | 1 | 62 | 0.040 |
| 2 | 63 | 0.040 | |
| 3 | 75 | 0.048 | |
| NT64 | 1 | 62 | 0.039 |
| 2 | 66 | 0.041 | |
| 3 | 22 | 0.012 | |
| 4 | 83 | 0.052 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szkoda, M.; Trzciński, K.; Zarach, Z.; Roda, D.; Łapiński, M.; Nowak, A.P. Scaling Up the Process of Titanium Dioxide Nanotube Synthesis and Its Effect on Photoelectrochemical Properties. Materials 2021, 14, 5686. https://doi.org/10.3390/ma14195686
Szkoda M, Trzciński K, Zarach Z, Roda D, Łapiński M, Nowak AP. Scaling Up the Process of Titanium Dioxide Nanotube Synthesis and Its Effect on Photoelectrochemical Properties. Materials. 2021; 14(19):5686. https://doi.org/10.3390/ma14195686
Chicago/Turabian StyleSzkoda, Mariusz, Konrad Trzciński, Zuzanna Zarach, Daria Roda, Marcin Łapiński, and Andrzej P. Nowak. 2021. "Scaling Up the Process of Titanium Dioxide Nanotube Synthesis and Its Effect on Photoelectrochemical Properties" Materials 14, no. 19: 5686. https://doi.org/10.3390/ma14195686