Assessment of the Potential Ability to Penetrate into the Hard Tissues of the Root of an Experimental Preparation with the Characteristics of a Dental Infiltratant, Enriched with an Antimicrobial Component—Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Demineralization of Tooth Root Cement
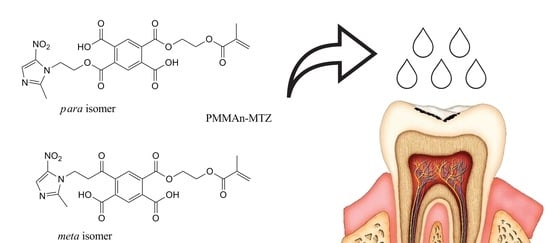
2.2. Preparation of the Experimental Solution
2.3. Tooth Infiltration Procedure
2.4. Preparation of Samples for Microscopic Analysis
2.5. Microscope Observations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Frecken, J. Caries Epidemiology and Its Challenges. Monogr. Oral Sci. 2018, 27, 11–23. [Google Scholar]
- Conrads, G.; About, I. Pathophysiology of Dental Caries. Monogr. Oral Sci. 2018, 27, 1–10. [Google Scholar]
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, A.; et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef]
- Mathur, V.P.; Dhillon, J.K. Dental Caries: A Disease Which Needs Attention. Indian J. Pediatr. 2018, 3, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Wójcicka, A.; Zalewska, M.; Czerech, E.; Jabłoński, R.; Grabowska, S.Z.; Maciorkowska, E. Próchnica Wieku Rozwojowego Chorobą Cywilizacyjną. Przegl Epidemiol. 2012, 66, 705–711. [Google Scholar] [PubMed]
- Giacaman, R.A.; Muñoz-Sandoval, C.; Neuhaus, K.W.; Fontana., M.; Chałas, R. Evidence-based strategies for the minimally invasive treatment of carious lesions: Review of the literature. Adv. Clin. Exp. Med. 2018, 7, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Wierichs, R.J.; Meyer-Lueckel, H. Systematic review on noninvasive treatment of root caries lesions. J. Dent. Res. 2015, 2, 261–271. [Google Scholar] [CrossRef]
- Tanasiewicz, M.; Skucha-Nowak, M.; Skorus, M.; Nowak, M. Stomatologia minimalnie inwazyjna. TPS 2018, 3, 23–26. [Google Scholar]
- Kaczmarek, U. Minimal intervention Dentistry—Review of literature. Czas. Stomatol. 2007, 6, 367–376. [Google Scholar]
- Dawett, B.; Young, S.; Deery, C.; Banerjee, A. Minimally Invasive Selective Caries Removal put into Practice. Dent. Update 2020, 47, 10. [Google Scholar] [CrossRef]
- Showkat, N.; Singh, G.; Singla, K.; Sareen, K.; Chowdhury, C.; Jindal, L. Minimal Invasive Dentistry: Literature Review. J. CMRO 2020, 9, 631–636. [Google Scholar]
- Ekstrand, K.R.; Gimenez, T.; Ferreira, F.R.; Mendes, F.M.; Braga, M.M. The International Caries Detection and Assessment System—ICDAS: A Systematic Review. Caries Res. 2018, 5, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Litzenburger, F.; Schäfer, G.; Hickel, R.; Kühnisch, F.; Heck, K. Comparison of novel and established caries diagnostic methods: A clinical study on occlusal surfaces. BMC Oral Health 2021, 21, 79. [Google Scholar] [CrossRef]
- Mitchell, C.; Zaku, H.; Milgrom, P.; Mancl, L.; Prince, D.B. The accuracy of laser fluorescence (DIAGNOdent) in assessing caries lesion activity on root surfaces, around crown margins, and in furcations in older adults. BDJ Open 2021, 23, 1–5. [Google Scholar]
- Abogazalah, N.; Ando, M. Alternative methods to visual and radiographic examinations for approximal caries detection. J. Oral Sci. 2017, 59, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Berczyński, P.; Gmerek, A.; Buczkowska-Radlińska, J. Remineralizing methods in early caries Lesions—Review of the liteerature. Pom. J. Life Sci. 2015, 61, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Arifa, M.K.; Ephraim, R.; Rajamani, T. Recent Advances in Dental Hard Tissue Remineralization: A Review of Literature. Int J Clin. Pediatr. Dent. 2019, 2, 139–144. [Google Scholar] [CrossRef]
- Sivapriya, E.; Sridevi, K.; Periasamy, R.; Lakshminarayanan, L.; Pradeepkumar, A.R. Remineralization ability of sodium fluoride on the microhardness of enamel, dentin, and dentinoenamel junction: An in vitro study. J. Conserv. Dent. 2017, 2, 100–104. [Google Scholar]
- Chen, Y.; Chen, D.; Lin, H. Infiltration and sealing for managing non-cavitated proximal lesions: A systematic review and meta-analysis. BMC Oral Health 2021, 21, 13. [Google Scholar] [CrossRef]
- Skucha-Nowak, M.; Fischer, M.; Nowak, M.; Łopaciński, M.; Tanasiewicz, M. Infiltracja odwapnionego szkliwa jako sposób leczenia próchnicy. Med. Trib. Stomatol. 2019, 4, 5–10. [Google Scholar]
- Zakizade, M.; Davoudi, A.; Akhavan, A.; Shirban, F. Effect of Resin Infiltration Technique on Improving Surface Hardness of Enamel Lesions: A Systematic Review and Meta-analysis. J. Evid. Based Dent. Pract. 2020, 2, 101405. [Google Scholar] [CrossRef]
- Kajka-Hawryluk, K.; Furmaniak, K.; Gromak-Zaremba, J.; Szopiński, K. Bitewing radiography in modern pediatric dentistry. Nowa Stomatol. 2015, 2, 73–80. [Google Scholar] [CrossRef]
- Ozyurt, E.; Arisu, H.D.; Turkoz, E. In Vitro Comparison of the Effectiveness of a Resin Infiltration Systemand a Dental Adhesive System in Dentinal Tubule Penetration. Clin. Exp. Health Sci. 2019, 9, 253–260. [Google Scholar]
- Diago, A.M.; Cadenaro, M.; Ricchiuto, R.; Banchelli, F.; Spinas, E.; Checchi, V.; Giannetti, L. Hypersensitivity in Molar Incisor Hypomineralization: Superficial Infiltration Treatment. Appl. Sci. 2021, 11, 1823. [Google Scholar] [CrossRef]
- Skucha-Nowak, M.; Machorowska-Pieniążek, A.; Tanasiewicz, M. Assesing the Penetrating Abilities of Experimental Preparation with Dental Infiltrant Features Using Optical Microscope: Preliminary Study. Adv. Clin. Exp. Med. 2016, 25, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Mertas, A.; Czuba, Z.P.; Skucha-Nowak, M. Study of cytotoxic properties of an experimental preparation 2 with features of a dental infiltrant. Materials 2021, 14, 2442. [Google Scholar] [CrossRef]
- Barczak, K.; Palczewska-Komsa, M.; Buczkowska-Radlińska, J. Physiological and pathological changes in the teeth and periodontal tissues related to age. GERIATRIA 2016, 10, 98–104. [Google Scholar]
- Yamamoto, T.; Hasegawa, T.; Yamamoto, T.; Hongo, H.; Amizuka, N. Histology of human cementum: Its structure, function, and development. Jpn. Dent. Sci. Rev. 2016, 52, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Prymas, A.; Wędrychowicz-Welman, A.; Mania-Końsko, A. Root Caries Treatment –Clinical Case. Dent. Med. Probl. 2006, 1, 135–138. [Google Scholar]
- Gernhardt, C.R. Wurzelkaries—Ein Problem im Alter Eine Übersicht über Ätiologie, Epidemiologie und das klinische Erscheinungsbild kariöser Läsionen im Wurzelbereich. Art Dent. 2018, 67, 22–29. [Google Scholar]
- Dinakaran, S.; Gopinathan, A.S. Root caries: A geriatric challenge. Dent. Med. Probl. 2017, 4, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Katz, R.V.; Hazen, S.P.; Chilton, N.W.; Mumma, R.D. Prevalence and intraoral distribution of root caries in an adult population. Caries Res. 1982, 16, 265–271. [Google Scholar] [CrossRef]
- Składnik-Jankowska, J.; Pregiel, B.; Wrzyszcz-Kowalczyk, A.; Kaczmarek, U. Management of Dental Root Caries Using Ozone. Dent. Med. Probl. 2005, 2, 273–279. [Google Scholar]
- Shay, K. Root caries in the older patient. Dent. Clin. N. Am. 1997, 41, 763–793. [Google Scholar]
- Ravald, N.; Birkhed, D. Factors associated with active and inactive root caries in patients with periodontal disease. Caries Res. 1991, 25, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Hryncewicz, M.; Tropak, K. Non-carious lesions—abfraction, abrasion, attrition, erosion. Review of literature. Borgis Nowa Stomatol. 2014, 1, 46–52. [Google Scholar]
- Collares, F.M.; Garcia, I.M.; Bohns, F.R.; Melo, M.A.; Branco Leitune, V.C. Guanidine hydrochloride polymer additive to 563 undertake ultraconservative resin infiltrant against Streptococcus mutans. Eur. Polym. J. 2020, 133, 109746. [Google Scholar] [CrossRef]
- Skucha-Nowak, M.; Mertas, A.; Tanasiewicz, M. Using an Electron Scanning Microscope to Assess the Penetrating Abilities of an Experimental Preparation with Features of a Dental Infiltrant: Preliminary Study. Adv. Clin. Exp. Med. 2016, 6, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Rusyan, E. Etiology and modifying factors of dental erosion. Borgis—Nowa Stomatol. 2003, 1, 33–36. [Google Scholar]
- Skucha-Nowak, M.; Tanasiewicz, M.; Gibas, M.; Twardawa, H. Analysis of the composition of preparations used as a barrier 544 to protect tissues of the patient against the influence of the environment in the oral cavity. Pol. J. Environ. Stud. 2013, 22, 53–57. [Google Scholar]
- Vespa, J.; Medina, L.; Armstrong, D.M. Demographic Turning Points for the United States: Population Projections for 2020 to 2060 Population Estimates and Projections; U.S. Department of Commerce, U.S. Census Bureau: Washington, DC, USA, 2018. Available online: http://www.census.gov/ (accessed on 15 March 2018).
- Statistics Poland. Ludność. Stan I Struktura Oraz Ruch Naturalny W Przekroju Terytorialnym W 2020 R. Stan W Dniu 31 XII. Population. Size and Structure and Vital Statistics in Poland by Territorial Division in 2020. As of 31 December; Demographic Surveys Department: Warszawa, Poland, 2021. [Google Scholar]
- Konopka, T.; Zawada, Ł.; Kobierzycka, A.; Chrzęszczyk, D. Periodontal Condition in 35–44 and 65–74 Year-Old Residents from Lower Silesia Region. Dent. Med. Probl. 2015, 4, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Gati, D.; Vieira, A.R. Elderly at greater risk for root caries: A look at the multifactorial risks with emphasis on genetics susceptibility. Int. J. Dent. 2011, 2011, 647168. [Google Scholar] [CrossRef] [Green Version]
- Gavriilidou, N.N.; Belibasakis, G.N. Root caries: The intersection between periodontal disease and dental caries in the course of ageing. Br. Dent. J. 2019, 12, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Theophilus, L.V.; Kida Minja, I.; Lembariti, B.S. Root Caries Prevalence and Associated Socio-Behavioral and Clinical Factors Among Elderly Patients Attending Selected Public Dental Clinics in Dar Es Salaam, Tanzania. J. Dent. Oral Sci. 2021, 1, 11–12. [Google Scholar]
- Strassler, H.E. Cervical Caries—Treatment Options Based Upon Etiology of the Lesion. Inside Dentistry 2005, 1, 1. [Google Scholar]
- Slavkin, H.C. Maturity and oral health: Live longer and better. J. Am. Dent. Assoc. 2000, 6, 805–808. [Google Scholar] [CrossRef]
- Hellyer, P.H.; Beighton, D.; Heath, P.; Lynch, E.J. Root caries in older people attending a general dental practice in East Sussex. Br. Dent. J. 1990, 6, 201–206. [Google Scholar] [CrossRef]
- Scribante, A.; Poggio, C.; Gallo, S.; Riva, P.; Cuocci, A.; Carbone, M.; Arciola, C.R.; Colombo, M. In Vitro Re-Hardening of Bleached Enamel Using Mineralizing Pastes: Toward Preventing Bacterial Colonization. Materials 2020, 4, 818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Matin, K.; Shimada, Y.; Sumi, Y.; Tagami, J. Evaluation of resin infiltration on demineralized root surface: An in vitro study. Dent. Mater. J. 2017, 2, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Yazkan, B.; Ermis, B. Effect of resin infiltration and microabrasion on the microhardness, surface roughness and morphology of incipient carious lesions. Acta Odontol. Scand. 2018, 76, 473–481. [Google Scholar] [CrossRef]
- Gurdogan, E.B.; Ozdemir-Ozenen, D.; Sandalli, N. Evaluation of Surface Roughness Characteristics Using Atomic Force Microscopy and Inspection of Microhardness Following Resin Infiltration with Icon®. JERD 2017, 3, 201–208. [Google Scholar] [CrossRef]
- Skucha-Nowak, M. Attempt to assess the infiltration of enamel made with experimental preparation using a scanning electron microscope. Open Med. Former. Cent. Eur. J. Med. 2015, 1, 238–248. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Zhou, X. Effect of Antibacterial Dental Adhesive on Multispecies Biofilms Formation. J. Dent. Res. 2015, 4, 622–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neres, E.; Moda, M.D.; Chiba, E.K.; Briso, A.; Pessan, J.P.; Fagundes, T.C. Microhardness and Roughness of Infiltrated White Spot Lesions Submitted to Different Challenges. Oper. Dent. 2017, 4, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, P.; Babu, G.; Lakhotia, D. Evaluation of penetration depth of a commercially available resin infiltrate into artificially created enamel lesions: An in vitro study. J. Conserv. Dent. 2014, 2, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, A.M.; Müller, A.; Gerhard, C.R. Closing the gap between oral higiene and minimally invasive infiltrationtechnique of incipient (proximal) enamel lesions. Quintessence Int. 2009, 40, 663–681. [Google Scholar] [PubMed]
- Tosco, V.; Vitiello, F.; Furlani, M.; Gatto, M.L.; Monterubbianesi, R.; Giuliani, A.; Orsini, G.; Putignano, A. Microleakage Analysis of Different Bulk-Filling Techniques for Class II Restorations: µ-CT, SEM and EDS Evaluations. Materials 2021, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Putignano, A.; Tosco, V.; Monterubbianesi, R.; Vitiello, A.; Gatto, M.L.; Furlani, M.; Giuliani., A.; Orsini, G. Comparison of three different bulk-filling techniques for restoring class II cavities: μCT, SEM-EDS combined analyses for margins and internal fit assessments. J. Mech. Behav. Biomed. Mater. 2021, 124, 104812. [Google Scholar] [CrossRef]







| Component | Molar Concentration | Quantity |
|---|---|---|
| CaCl2*2H2O | 3 mM | 0.441 g |
| KH2PO4 | 3 mM | 0.408 g |
| CH3COOH | 50 mM | 2.88 mL |
| MHDP | 6 µM | 1.416 × 10−3 g |
| Component | Quantity (g) | Content (%) |
|---|---|---|
| TEGDMA | 3.75 | 75 |
| HEMA | 1.25 | 25 |
| PMMAn-MTZ 1 | 0.05 | 1 * |
| DMAEMA 2 | 0.05 | 1 * |
| CQ 3 | 0.025 | 0.5 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, M.; Skucha-Nowak, M.; Chmiela, B.; Korytkowska-Wałach, A. Assessment of the Potential Ability to Penetrate into the Hard Tissues of the Root of an Experimental Preparation with the Characteristics of a Dental Infiltratant, Enriched with an Antimicrobial Component—Preliminary Study. Materials 2021, 14, 5654. https://doi.org/10.3390/ma14195654
Fischer M, Skucha-Nowak M, Chmiela B, Korytkowska-Wałach A. Assessment of the Potential Ability to Penetrate into the Hard Tissues of the Root of an Experimental Preparation with the Characteristics of a Dental Infiltratant, Enriched with an Antimicrobial Component—Preliminary Study. Materials. 2021; 14(19):5654. https://doi.org/10.3390/ma14195654
Chicago/Turabian StyleFischer, Małgorzata, Małgorzata Skucha-Nowak, Bartosz Chmiela, and Anna Korytkowska-Wałach. 2021. "Assessment of the Potential Ability to Penetrate into the Hard Tissues of the Root of an Experimental Preparation with the Characteristics of a Dental Infiltratant, Enriched with an Antimicrobial Component—Preliminary Study" Materials 14, no. 19: 5654. https://doi.org/10.3390/ma14195654