Ag/MgO Nanoparticles via Gas Aggregation Nanocluster Source for Perovskite Solar Cell Engineering
Abstract
:1. Introduction
2. Materials and Methods
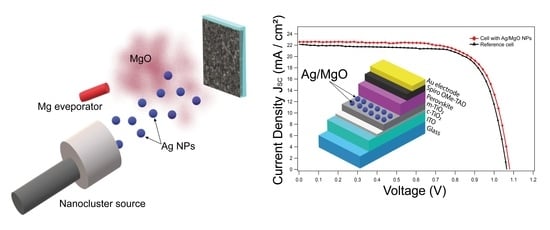
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiency Chart|Photovoltaic Research|NREL. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 26 March 2021).
- Webb, T.; Sweeney, S.J.; Zhang, W. Device Architecture Engineering: Progress toward Next Generation Perovskite Solar Cells. Adv. Funct. Mater. 2021, 2103121. [Google Scholar] [CrossRef]
- Chen, J.; Park, N. Materials and Methods for Interface Engineering toward Stable and Efficient Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 2742–2786. [Google Scholar] [CrossRef]
- Roose, B.; Wang, Q.; Abate, A. The Role of Charge Selective Contacts in Perovskite Solar Cell Stability. Adv. Energy Mater. 2019, 9, 1803140. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, Y.; Ge, Z. Chem Soc Rev Understanding of perovskite crystal growth and film formation in scalable deposition processes. Chem. Soc. Rev 2020, 49, 1653–1687. [Google Scholar] [CrossRef] [PubMed]
- Thrithamarassery Gangadharan, D.; Ma, D. Searching for stability at lower dimensions: Current trends and future prospects of layered perovskite solar cells. Energy Environ. Sci. 2019, 12, 2860–2889. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Schmidt-Mende, L.; Garcia-Belmonte, G.; Jose, R.; Mora-Sero, I. Interfaces in perovskite solar cells. Adv. Electron. Mater. 2017, 7, 1–44. [Google Scholar] [CrossRef]
- Giordano, F.; Abate, A.; Pablo, J.; Baena, C.; Saliba, M.; Matsui, T.; Im, S.H.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Hagfeldt, A.; et al. Enhanced electronic properties in mesoporous TiO2 via lithium doping for high-efficiency perovskite solar cells. Nat. Commun. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Amalathas, A.P.; Landov, L.; Huminiuc, T. Elucidating the role of TiCl4 post-treatment on percolation of TiO2 electron transport layer in perovskite solar cells. J. Phys. D Appl. Phys. 2020, 53, 385501. [Google Scholar] [CrossRef]
- Zhen, C.; Wu, T.; Chen, R.; Wang, L.; Liu, G.; Cheng, H. Strategies for Modifying TiO2 Based Electron Transport Layers to Boost Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2019, 7, 4586–4618. [Google Scholar] [CrossRef]
- Di Carlo, A.; Agresti, A.; Brunetti, F.; Pescetelli, S. Two-dimensional materials in perovskite solar cells. J. Phys. Energy 2020, 2, 031003. [Google Scholar] [CrossRef]
- Taheri, B.; Nia, N.Y.; Agresti, A.; Pescetelli, S.; Ciceroni, C.; Del Rio Castillo, A.E.; Cinà, L.; Bellani, S.; Bonaccorso, F.; Di Carlo, A. Graphene-engineered automated sprayed mesoscopic structure for perovskite device scaling-up. 2D Mater. 2018, 5, 045034. [Google Scholar] [CrossRef]
- Agresti, A.; Pescetelli, S.; Taheri, B.; Del Rio Castillo, A.E.; Cinà, L.; Bonaccorso, F.; Di Carlo, A. Graphene-Perovkite solar cells exceed 18% efficiency: A stability study. ChemSusChem 2016, 9, 2609–2619. [Google Scholar] [CrossRef] [PubMed]
- Agresti, A.; Pescetelli, S.; Palma, A.L.; Martin-Garcia, B.; Najafi, L.; Bellani, S.; Moreels, I.; Prato, M.; Bonaccorso, F.; Di Carlo, A. Two-dimensional (2D) Material Interface Engineering for Efficient Perovskite Large-area Modules. ACS Energy Lett. 2019, 4, 1862–1871. [Google Scholar] [CrossRef]
- Biccari, F.; Gabelloni, F.; Burzi, E.; Gurioli, M.; Pescetelli, S.; Agresti, A.; Del Rio Castillo, A.E.; Ansaldo, A.; Kymakis, E.; Bonaccorso, F.; et al. Graphene-Based Electron Transport Layers in Perovskite Solar Cells: A Step-Up for an Efficient Carrier Collection. Adv. Energy Mater. 2017, 7, 1701349. [Google Scholar] [CrossRef]
- Agresti, A.; Pescetelli, S.; Cinà, L.; Konios, D.; Kakavelakis, G.; Kymakis, E.; Carlo, A. Di Efficiency and Stability Enhancement in Perovskite Solar Cells by Inserting Lithium-Neutralized Graphene Oxide as Electron Transporting Layer. Adv. Funct. Mater. 2016, 26, 2686–2694. [Google Scholar] [CrossRef]
- Agresti, A.; Pescetelli, S.; Najafi, L.; Castillo, A.E.D.R.; Busby, Y.; Carlo, A. Di Graphene and related 2D materials for high efficient and stable perovskite solar cells. In Proceedings of the 17th IEEE International Conference on Nanotechnology Pittsburgh, Pittsburgh, PA, USA, 25–28 July 2017; pp. 145–150. [Google Scholar]
- Agresti, A.; Pazniak, A.; Pescetelli, S.; Di Vito, A.; Rossi, D.; Pecchia, A.; Auf der Maur, M.; Liedl, A.; Larciprete, R.; Kuznetsov, D.V.; et al. Titanium-carbide MXenes for work function and interface engineering in perovskite solar cells. Nat. Mater. 2019, 18, 1228–1234. [Google Scholar] [CrossRef] [Green Version]
- Saranin, D.; Pescetelli, S.; Pazniak, A.; Rossi, D.; Liedl, A.; Yakusheva, A.; Luchnikov, L.; Podgorny, D.; Gostischev, P.; Didenko, S.; et al. Transition metal carbides (MXenes) for efficient NiO-based inverted perovskite solar cells. Nano Energy 2021, 82, 105771. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef] [Green Version]
- Del Rio Castillo, A.E.; Pellegrini, V.; Ansaldo, A.; Ricciardella, F.; Sun, H.; Marasco, L.; Buha, J.; Dang, Z.; Gagliani, L.; Lago, E.; et al. High-yield production of 2D crystals by wet-jet milling. Mater. Horizons 2018, 5, 890–904. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Kou, Z.; Feng, J.; Sun, H. Plasmon-enhanced organic and perovskite solar cells with metal nanoparticles. Nanophotonics 2020, 9, 3111–3133. [Google Scholar] [CrossRef]
- Astruc, D. Nanoparticles and Catalysis; Wiley-VCH: Weinheim, Germany, 2008; ISBN 9783527315727. [Google Scholar]
- Westphalen, M.; Kreibig, U.; Rostalski, J.; Lüth, H.; Meissner, D. Metal cluster enhanced organic solar cells. Sol. Energy Mater. Sol. Cells 2000, 61, 97–105. [Google Scholar] [CrossRef]
- Derkacs, D.; Lim, S.H.; Matheu, P.; Mar, W.; Yu, E.T. Improved performance of amorphous silicon solar cells via scattering from surface plasmon polaritons in nearby metallic nanoparticles. Appl. Phys. Lett. 2006, 89, 093103. [Google Scholar] [CrossRef] [Green Version]
- Pillai, S.; Catchpole, K.R.; Trupke, T.; Green, M.A. Surface plasmon enhanced silicon solar cells. J. Appl. Phys. 2007, 101, 093105. [Google Scholar] [CrossRef]
- Catchpole, K.R.; Polman, A. Design principles for particle plasmon enhanced solar cells. Appl. Phys. Lett. 2008, 93, 191113. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.S.; Na, S.I.; Jo, J.; Kim, D.Y.; Nah, Y.C. Plasmon enhanced performance of organic solar cells using electrodeposited Ag nanoparticles. Appl. Phys. Lett. 2008, 93, 073307. [Google Scholar] [CrossRef]
- Park, J.; Park, N.; Varlamov, S. Optimum surface condition for plasmonic Ag nanoparticles in polycrystalline silicon thin film solar cells. Appl. Phys. Lett. 2014, 104, 033903. [Google Scholar] [CrossRef]
- Yao, K.; Zhong, H.; Liu, Z.; Xiong, M.; Leng, S.; Zhang, J.; Xu, Y.X.; Wang, W.; Zhou, L.; Huang, H.; et al. Plasmonic Metal Nanoparticles with Core-Bishell Structure for High-Performance Organic and Perovskite Solar Cells. ACS Nano 2019, 13, 5397–5409. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Hao, H.; Li, J.; Shi, L.; Zhong, T.; Zhang, C.; Dong, J.; Xing, J.; Liu, H.; Zhang, Z. Light trapping effect in perovskite solar cells by the addition of ag nanoparticles, using textured substrates. Nanomaterials 2018, 8, 815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Li, B.Q.; Wang, W. Forward scattering nanoparticles based nanostructure for light trapping over solar spectrum. AIP Adv. 2019, 9, 085119. [Google Scholar] [CrossRef]
- Yuan, Z.; Wu, Z.; Bai, S.; Xia, Z.; Xu, W.; Song, T.; Wu, H.; Xu, L.; Si, J.; Jin, Y.; et al. Hot-Electron Injection in a Sandwiched TiOx-Au-TiOx Structure for High-Performance Planar Perovskite Solar Cells. Adv. Energy Mater. 2015, 5, 1500038. [Google Scholar] [CrossRef]
- Furube, A.; Du, L.; Hara, K.; Katoh, R.; Tachiya, M. Ultrafast plasmon-induced electron transfer from gold nanodots into TiO2 nanoparticles. J. Am. Chem. Soc. 2007, 129, 14852–14853. [Google Scholar] [CrossRef] [PubMed]
- Giugni, A.; Torre, B.; Toma, A.; Francardi, M.; Malerba, M.; Alabastri, A.; Proietti Zaccaria, R.; Stockman, M.I.; Di Fabrizio, E. Hot-electron nanoscopy using adiabatic compression of surface plasmons. Nat. Nanotechnol. 2013, 8, 845–852. [Google Scholar] [CrossRef]
- Dong, H.; Lei, T.; Yuan, F.; Xu, J.; Niu, Y.; Jiao, B.; Zhang, Z.; Ding, D.; Hou, X.; Wu, Z. Plasmonic enhancement for high efficient and stable perovskite solar cells by employing “hot spots” Au nanobipyramids. Org. Electron. 2018, 60, 1–8. [Google Scholar] [CrossRef]
- Solzi, M.; Pernechele, C.; Calestani, G.; Villani, M.; Gaboardi, M.; Migliori, A. Non-interacting hard ferromagnetic L10 FePt nanoparticles embedded in a carbon matrix. J. Mater. Chem. 2011, 21, 18331–18338. [Google Scholar] [CrossRef]
- Svanström, S.; Jacobsson, T.J.; Boschloo, G.; Johansson, E.M.J.; Rensmo, H.; Cappel, U.B. Degradation Mechanism of Silver Metal Deposited on Lead Halide Perovskites. ACS Appl. Mater. Interfaces 2020, 12, 7212–7221. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Zhang, C.; Deng, X.; Zhu, H.; Li, Z.; Wang, Z.; Chen, X.; Huang, S. Plasmonic Effects of Metallic Nanoparticles on Enhancing Performance of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 34821–34832. [Google Scholar] [CrossRef]
- Kluczyk-Korch, K.; David, C.; Jacak, W.; Jacak, J. Application of core-shell metallic nanoparticles in hybridized perovskite solar cell-various channels of plasmon photovoltaic effect. Materials 2019, 12, 3192. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Saliba, M.; Stranks, S.D.; Sun, Y.; Shi, X.; Wiesner, U.; Snaith, H.J. Enhancement of perovskite-based solar cells employing core-shell metal nanoparticles. Nano Lett. 2013, 13, 4505–4510. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, R.; Xue, J.; Xing, X.; Yu, C.; Huang, T.; Chu, J.; Wang, K.L.; Dong, C.; Wei, Z.; et al. Core-Shell ZnO@SnO2 Nanoparticles for Efficient Inorganic Perovskite Solar Cells. J. Am. Chem. Soc. 2019, 141, 17610–17616. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, X.; Li, S.; Chen, M.; Liu, N.; Yang, H.; Ran, M.; Lu, H.; Yang, Y. Enhancing the Photovoltaic Performance of Perovskite Solar Cells Using Plasmonic Au@Pt@Au Core-Shell Nanoparticles. Nanomaterials 2019, 9, 1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Pan, X.; Ma, Y.; Li, Y.; Zheng, L.; Zhang, D.; Xu, Q.; Chen, Z.; Wang, S.; Qu, B.; et al. Plasmonic-enhanced perovskite solar cells using alloy popcorn nanoparticles. RSC Adv. 2015, 5, 11175–11179. [Google Scholar] [CrossRef]
- Ginting, R.T.; Kaur, S.; Lim, D.K.; Kim, J.M.; Lee, J.H.; Lee, S.H.; Kang, J.W. Plasmonic Effect of Gold Nanostars in Highly Efficient Organic and Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 36111–36118. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 1995; Volume 25, ISBN 978-3-642-08191-0. [Google Scholar]
- Saliba, M.; Zhang, W.; Burlakov, V.M.; Stranks, S.D.; Sun, Y.; Ball, J.M.; Johnston, M.B.; Goriely, A.; Wiesner, U.; Snaith, H.J. Plasmonic-Induced Photon Recycling in Metal Halide Perovskite Solar Cells. Adv. Funct. Mater. 2015, 25, 5038–5046. [Google Scholar] [CrossRef]
- Kumar, V.S.S.; Rao, K.V. Synthesis and study of ultrasonic parameters of MgO-ethylene glycol nanofluids. J. Nanofluids 2018, 7, 269–274. [Google Scholar] [CrossRef]
- D’Addato, S.; Pinotti, D.; Spadaro, M.C.; Paolicelli, G.; Grillo, V.; Valeri, S.; Pasquali, L.; Bergamini, L.; Corni, S. Influence of size, shape and core-shell interface on surface plasmon resonance in Ag and Ag@MgO nanoparticle films deposited on Si/SiOx. Beilstein J. Nanotechnol. 2015, 6, 404–413. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Luo, Q.; Shi, J.; Yue, L.; Wang, Z.; Chen, X.; Huang, S. Efficient perovskite solar cells by combination use of Au nanoparticles and insulating metal oxide. Nanoscale 2017, 9, 2852–2864. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Qin, M.; Tao, H.; Ke, W.; Chen, Z.; Wan, J.; Qin, P.; Xiong, L.; Lei, H.; Yu, H.; et al. Performance enhancement of perovskite solar cells with Mg-doped TiO2 compact film as the hole-blocking layer. Appl. Phys. Lett. 2015, 106, 121104. [Google Scholar] [CrossRef]
- Spadaro, M.C.; D’Addato, S.; Luches, P.; Valeri, S.; Grillo, V.; Rotunno, E.; Roldan, M.A.; Pennycook, S.J.; Ferretti, A.M.; Capetti, E.; et al. Tunability of exchange bias in Ni@NiO core-shell nanoparticles obtained by sequential layer deposition. Nanotechnology 2015, 26, 405704. [Google Scholar] [CrossRef] [PubMed]
- D’Addato, S.; Gunnella, R.; Borgatti, F.; Felici, R.; Finetti, P. Atom geometry of nanostructured Fe films grown on c(2 × 2)-N/Cu(1 0 0) surface: An investigation by X-ray absorption spectroscopy with multishell analysis. Surf. Sci. 2007, 601, 329–340. [Google Scholar] [CrossRef]
- Bansmann, J.; Baker, S.H.; Binns, C.; Blackman, J.A.; Bucher, J.P.; Dorantes-Dávila, J.; Dupuis, V.; Favre, L.; Kechrakos, D.; Kleibert, A.; et al. Magnetic and structural properties of isolated and assembled clusters. Surf. Sci. Rep. 2005, 56, 189–275. [Google Scholar] [CrossRef] [Green Version]
- Llamosa, D.; Ruano, M.; Martínez, L.; Mayoral, A.; Roman, E.; García-Hernández, M.; Huttel, Y. The ultimate step towards a tailored engineering of core@shell and core@shell@shell nanoparticles. Nanoscale 2014, 6, 13483–13486. [Google Scholar] [CrossRef] [PubMed]
- Razza, S.; Pescetelli, S.; Agresti, A.; Carlo, A. Di Laser Processing Optimization for Large-Area Perovskite Solar Modules. Energies 2021, 14, 1069. [Google Scholar] [CrossRef]
- Larciprete, R.; Agresti, A.; Pescetelli, S.; Pazniak, H.; Liedl, A.; Lacovig, P.; Lizzit, D.; Tosi, E.; Lizzit, S.; Carlo, A. Di Physical Stress. Materials 2021, 14, 3954. [Google Scholar] [CrossRef]
- Polyakov, A.Y.; Smirnov, N.B.; Shchemerov, I.V.; Saranin, D.S.; Le, T.S.; Didenko, S.I.; Kuznetsov, D.V.; Agresti, A.; Pescetelli, S.; Matteocci, F.; et al. Trap states in multication mesoscopic perovskite solar cells: A deep levels transient spectroscopy investigation. Appl. Phys. Lett. 2018, 113, 263501. [Google Scholar] [CrossRef]
- D’Addato, S.; Grillo, V.; Altieri, S.; Tondi, R.; Valeri, S.; Frabboni, S. Structure and stability of nickel/nickel oxide core-shell nanoparticles. J. Phys. Condens. Matter 2011, 23, 175003. [Google Scholar] [CrossRef]
- D’Addato, S.; Grillo, V.; Di Bona, A.; Luches, P.; Frabboni, S.; Valeri, S.; Lupo, P.; Casoli, F.; Albertini, F. Controlled co-deposition of FePt nanoparticles embedded in MgO: A detailed investigation of structure and electronic and magnetic properties. Nanotechnology 2013, 24, 495703. [Google Scholar] [CrossRef] [PubMed]
- Lucas, G.; Burdet, P.; Cantoni, M.; Hébert, C. Multivariate statistical analysis as a tool for the segmentation of 3D spectral data. Micron 2013, 52, 49–56. [Google Scholar] [CrossRef]
- Agresti, A.; Cinà, L.; Pescetelli, S.; Taheri, B.; Di Carlo, A. Stability of dye-sensitized solar cell under reverse bias condition: Resonance Raman spectroscopy combined with spectrally resolved analysis by transmittance and efficiency mapping. Vib. Spectrosc. 2016, 84, 106–117. [Google Scholar] [CrossRef]
- Agresti, A.; Pescetelli, S.; Quatela, A.; Mastroianni, S.; Brown, T.M.; Reale, A.; Bignozzi, C.A.; Caramori, S.; Di Carlo, A. Micro-Raman analysis of reverse bias stressed dye-sensitized solar cells. RSC Adv. 2014, 4, 12366. [Google Scholar] [CrossRef]
- Agresti, A.; Berionni Berna, B.; Pescetelli, S.; Catini, A.; Menchini, F.; Di Natale, C.; Paolesse, R.; Di Carlo, A. Copper-Based Corrole as Thermally Stable Hole Transporting Material for Perovskite Photovoltaics. Adv. Funct. Mater. 2020, 30, 1–11. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.; Lee, B.; He, J.; Chang, R.P.H.; Kanatzidis, M.G. All-solid-state dye-sensitized solar cells with high efficiency. Nature 2012, 485, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Deepa, M.; Salado, M.; Calio, L.; Kazim, S.; Shivaprasad, S.M.; Ahmad, S. Cesium power: Low Cs+ levels impart stability to perovskite solar cells. Phys. Chem. Chem. Phys. 2017, 19, 4069–4077. [Google Scholar] [CrossRef] [PubMed]
- Kar, A. Nanoelectronics and Materials Development. Nanoelectron. Mater. Dev. 2016. [Google Scholar] [CrossRef]
- Higgins, M.; Ely, F.; Nome, R.C.; Nome, R.A.; dos Santos, D.P.; Choi, H.; Nam, S.; Quevedo-Lopez, M. Enhanced reproducibility of planar perovskite solar cells by fullerene doping with silver nanoparticles. J. Appl. Phys. 2018, 124, 065306. [Google Scholar] [CrossRef]
- Ma, J.; Yang, G.; Qin, M.; Zheng, X.; Lei, H.; Chen, C.; Chen, Z.; Guo, Y.; Han, H.; Zhao, X.; et al. MgO Nanoparticle Modified Anode for Highly Efficient SnO2-Based Planar Perovskite Solar Cells. Adv. Sci. 2017, 4, 1700031. [Google Scholar] [CrossRef]
- Marks, L.D. Experimental studies of small particle structures. Reports Prog. Phys. 1994, 57, 603–649. [Google Scholar] [CrossRef]
- Mackay, A.L. A dense non-crystallographic packing of equal spheres. Acta Crystallogr. 1962, 15, 916–918. [Google Scholar] [CrossRef]
- Ino, S. Stability of Multiply-Twinned Particles. J. Phys. Soc. Japan 1969, 27, 941–953. [Google Scholar] [CrossRef]
- Barke, I.; Hartmann, H.; Rupp, D.; Flückiger, L.; Sauppe, M.; Adolph, M.; Schorb, S.; Bostedt, C.; Treusch, R.; Peltz, C.; et al. The 3D-architecture of individual free silver nanoparticles captured by X-ray scattering. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kleibert, A.; Bulut, F.; Gebhardt, R.K.; Rosellen, W.; Sudfeld, D.; Passig, J.; Bansmann, J.; Meiwes-Broer, K.H.; Getzlaff, M. Correlation of shape and magnetic anisotropy of supported mass-filtered Fe and FeCo alloy nanoparticles on W(110). J. Phys. Condens. Matter 2008, 20, 445005. [Google Scholar] [CrossRef]
- Kleibert, A.; Rosellen, W.; Getzlaff, M.; Bansmann, J.; Wiedwald, U.; Ziemann, P. Structure, morphology, and magnetic properties of Fe nanoparticles deposited onto single-crystalline surfaces. Beilstein J. Nanotechnol. 2011, 2, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Ito, S.; Zakeeruddin, S.M.; Humphry-Baker, R.; Liska, P.; Charvet, R.; Comte, P.; Nazeeruddin, M.K.; Péchy, P.; Takata, M.; Miura, H.; et al. High-Efficiency Organic-Dye- Sensitized Solar Cells Controlled by Nanocrystalline-TiO2 Electrode Thickness. Adv. Mater. 2006, 18, 1202–1205. [Google Scholar] [CrossRef]
- Collins, T.J. ImageJ for microscopy. Biotechniques 2007, 43, S25–S30. [Google Scholar] [CrossRef]
- O’Grady, K.; Bradbury, A. Particle size analysis in ferrofluids. J. Magn. Magn. Mater. 1983, 39, 91–94. [Google Scholar] [CrossRef]
- D’Addato, S.; Gragnaniello, L.; Valeri, S.; Rota, A.; Di Bona, A.; Spizzo, F.; Panozaqi, T.; Schifano, S.F. Morphology and magnetic properties of size-selected Ni nanoparticle films. J. Appl. Phys. 2010, 107, 104318. [Google Scholar] [CrossRef]
- Getzlaff, M.; Bansmann, J.; Bulut, F.; Gebhardt, R.K.; Kleibert, A.; Meiwes-Broer, K.H. Structure, composition and magnetic properties of size-selected FeCo alloy clusters on surfaces. Appl. Phys. A Mater. Sci. Process. 2006, 82, 95–101. [Google Scholar] [CrossRef]
- Mesquita, I.; Andrade, L.; Mendes, A. Temperature Impact on Perovskite Solar Cells Under Operation. ChemSusChem 2019, 12, 2186–2194. [Google Scholar] [CrossRef]
- Pelli Cresi, J.S.; Silvagni, E.; Bertoni, G.; Spadaro, M.C.; Benedetti, S.; Valeri, S.; D’Addato, S.; Luches, P. Optical and electronic properties of silver nanoparticles embedded in cerium oxide. J. Chem. Phys. 2020, 152, 114704. [Google Scholar] [CrossRef]
- Stenzel, O. The Physics of Thin Film Optical Spectra—An Introduction|Olaf Stenzel; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Gibaud, A.; Vignaud, G. Specular reflectivity from smooth and rough surfaces. Lect. Notes Phys. 2009, 770, 85–131. [Google Scholar] [CrossRef]
- Simonsen, I.; Lazzari, R.; Jupille, J.; Roux, S. Numerical modeling of the optical response of supported metallic particles. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 61, 7722–7733. [Google Scholar] [CrossRef] [Green Version]
- Noguez, C. Optical properties of isolated and supported metal nanoparticles. Opt. Mater. 2005, 27, 1204–1211. [Google Scholar] [CrossRef] [Green Version]
- Granqvist, C.G.; Hunderi, O. Optical properties of ultrafine gold particles. Phys. Rev. B 1977, 16, 3513–3534. [Google Scholar] [CrossRef]
- Pascua, L.; Stavale, F.; Nilius, N.; Freund, H.J. Ag/ZnO hybrid systems studied with scanning tunnelling microscopy-based luminescence spectroscopy. J. Appl. Phys. 2016, 119, 095310. [Google Scholar] [CrossRef] [Green Version]
- Güney, H.; İskenderoğlu, D. Synthesis of MgO thin films grown by SILAR technique. Ceram. Int. 2018, 44, 7788–7793. [Google Scholar] [CrossRef]
- Shin, D.Y.; Kim, K.N. Synthesis of MgO Thin Film Deposited on Soda Lime Glass by Sol-Gel Process. Mater. Sci. Forum 2008, 569, 61–64. [Google Scholar] [CrossRef]
- Edward, P. Handbook of Optical Constants of Solids. Available online: http://store.elsevier.com/product.jsp?isbn=9780125444231 (accessed on 26 March 2021).
- Aadim, K.A. Preparation single layer of (MgO) as antireflection coating using PLD technique. Iraqi J. Phys. 2018, 16, 39–46. [Google Scholar] [CrossRef]
- Noguez, C. Surface plasmons on metal nanoparticles: The influence of shape and physical environment. J. Phys. Chem. C 2007, 111, 3606–3619. [Google Scholar] [CrossRef]
- Cha, H.; Lee, D.; Yoon, J.H.; Yoon, S. Plasmon coupling between silver nanoparticles: Transition from the classical to the quantum regime. J. Colloid Interface Sci. 2016, 464, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, X.; Wang, B.; Qiao, Y.; Liu, W.; Yang, H.; Liu, N.; Chen, M.; Lu, H.; Yang, Y. Influence of Ag Nanoparticles with Different Sizes and Concentrations Embedded in a TiO2 Compact Layer on the Conversion Efficiency of Perovskite Solar Cells. Nanoscale Res. Lett. 2018, 13, 210. [Google Scholar] [CrossRef] [Green Version]
- Moakhar, R.S.; Gholipour, S.; Masudy-Panah, S.; Seza, A.; Mehdikhani, A.; Riahi-Noori, N.; Tafazoli, S.; Timasi, N.; Lim, Y.-F.; Saliba, M. Recent Advances in Plasmonic Perovskite Solar Cells. Adv. Sci. 2020, 7, 1902448. [Google Scholar] [CrossRef]
- Ma, X.; Ma, B.; Yu, T.; Xu, X.; Zhang, L.; Wang, W.; Cao, K.; Deng, L.; Chen, S.; Huang, W. Indepth Studies on Working Mechanism of Plasmon-Enhanced Inverted Perovskite Solar Cells Incorporated with Ag@SiO2 Core-Shell Nanocubes. ACS Appl. Energy Mater. 2019, 2, 3605–3613. [Google Scholar] [CrossRef]
- Bao, Z.; Fu, N.; Qin, Y.; Lv, J.; Wang, Y.; He, J.; Hou, Y.; Jiao, C.; Chen, D.; Wu, Y.; et al. Broadband Plasmonic Enhancement of High-Efficiency Dye-Sensitized Solar Cells by Incorporating Au@Ag@SiO2 Core-Shell Nanocuboids. ACS Appl. Mater. Interfaces 2020, 12, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Juan, F.; Wu, Y.; Shi, B.; Wang, M.; Wang, M.; Xu, F.; Jia, J.; Wei, H.; Yang, T.; Cao, B. Plasmonic Au Nanooctahedrons Enhance Light Harvesting and Photocarrier Extraction in Perovskite Solar Cell. ACS Appl. Energy Mater. 2021, 4, 3201–3209. [Google Scholar] [CrossRef]
- Fan, R.; Wang, L.; Chen, Y.; Zheng, G.; Li, L.; Li, Z.; Zhou, H. Tailored Au@TiO2 nanostructures for the plasmonic effect in planar perovskite solar cells. J. Mater. Chem. A 2017, 5, 12034–12042. [Google Scholar] [CrossRef]








| Ag/MgO Coverage % | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| 0.0 | 21.6 ± 0.3 | 1.04 ± 0.03 | 71.1 ± 1.0 | 16.0 ± 0.4 |
| 1.5 | 22.1 ± 0.4 | 1.07 ± 0.03 | 71.5 ± 2.4 | 16.5 ± 0.8 |
| 3.2 | 21.6 ± 0.7 | 1.06 ± 0.02 | 69.7 ± 2.8 | 15.0 ± 1.2 |
| 8.7 | 12.0 ± 1.4 | 1.00 ± 0.03 | 60.2 ± 2.2 | 8.4 ± 1.0 |
| 10.0 | 7.3 ± 0.7 | 0.98 ± 0.01 | 50.9 ± 3.3 | 5.0 ± 0.9 |
| Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) | |
|---|---|---|---|---|
| Reference | 22.0 | 1.05 | 72.2 | 17.0 |
| Ag/MgO 1.5% | 22.6 | 1.08 | 71.9 | 17.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caleffi, M.; Mariani, P.; Bertoni, G.; Paolicelli, G.; Pasquali, L.; Agresti, A.; Pescetelli, S.; Di Carlo, A.; De Renzi, V.; D’Addato, S. Ag/MgO Nanoparticles via Gas Aggregation Nanocluster Source for Perovskite Solar Cell Engineering. Materials 2021, 14, 5507. https://doi.org/10.3390/ma14195507
Caleffi M, Mariani P, Bertoni G, Paolicelli G, Pasquali L, Agresti A, Pescetelli S, Di Carlo A, De Renzi V, D’Addato S. Ag/MgO Nanoparticles via Gas Aggregation Nanocluster Source for Perovskite Solar Cell Engineering. Materials. 2021; 14(19):5507. https://doi.org/10.3390/ma14195507
Chicago/Turabian StyleCaleffi, Matteo, Paolo Mariani, Giovanni Bertoni, Guido Paolicelli, Luca Pasquali, Antonio Agresti, Sara Pescetelli, Aldo Di Carlo, Valentina De Renzi, and Sergio D’Addato. 2021. "Ag/MgO Nanoparticles via Gas Aggregation Nanocluster Source for Perovskite Solar Cell Engineering" Materials 14, no. 19: 5507. https://doi.org/10.3390/ma14195507