Co0.5Mn0.5Fe2O4@PMMA Nanoparticles Promotes Preosteoblast Differentiation through Activation of OPN-BGLAP2-DMP1 Axis and Modulates Osteoclastogenesis under Magnetic Field Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Situ Synthesis of the Binary Co0.5Mn0.5Fe2O4@PMMA Hybrids
2.2. Characterization of Basic Physicochemical Properties of PMMA@Co0.5Mn0.5Fe2O4 Hybrids
2.3. Cell Lines
2.4. Magnetic Field (MF)
2.5. Cell Proliferation Assay
2.6. Morphology and Mitochondria Status Analysis
2.7. Gene Expression Analysis
2.8. Statistical Analysis
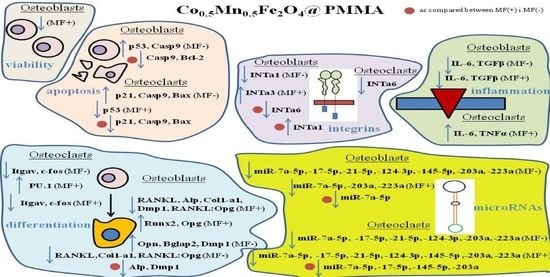
3. Results
3.1. Characterization of Physicochemical Properties of the Co0.5Mn0.5Fe2O4 Nanoparticles and PMMA@Co0.5Mn0.5Fe2O4
3.2. Anti-Proliferative Effect of PMMA Modified by Co0.5Mn0.5Fe2O4 in Ratio 80/20 towards Pre-Osteoblasts and Pre-Osteoclasts in the Presence of Magnetic Field
3.3. Morphology and Mitochondria Network Development Related to PMMA and PMMA@Co0.5Mn0.5Fe2O4 towards Osteoblasts and Osteoclasts in the Presence of Magnetic Field
3.4. The Impact of PMMA Modified by Co0.5Mn0.5Fe2O4 in Ratio 80/20 on the Expression of Genes Related to the Apoptosis towards Pre-Osteoblasts and Pre-Osteoclasts in the Presence of Magnetic Field
3.5. The Impact of PMMA Modified by Co0.5Mn0.5Fe2O4 in Ratio 80/20 on the Expression of Genes and Proteins Related to Osteogenesis/Osteoclastogenesiss towards Pre-Osteoblasts and Pre-Osteoclasts in the Presence of Magnetic Field
3.6. The Impact of PMMA Modified by Co0.5Mn0.5Fe2O4 in Ratio 80/20 on the Expression of Genes Related to Integrins towards Pre-Osteoblasts and Pre-Osteoclasts in the Presence of Magnetic Field
3.7. The Impact of PMMA Modified by Co0.5Mn0.5Fe2O4 in Ratio 80/20 on the Expression of Genes Related to Inflammation Process towards Pre-Osteoblasts and Pre-Osteoclasts in the Presence of Magnetic Field
3.8. The Impact of PMMA Modified by Co0.5Mn0.5Fe2O4 in Ratio 80/20 on the Expression of Genes Related to microRNA Involved in the Process of Osteoblastogenesis and Osteoclatogenesis towards Pre-Osteoblasts and Pre-Osteoclasts in the Presence of Magnetic Field
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| BAD | Bcl-2 associated agonist of cell death |
| BAX | Bcl-2 associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| BGLAP | bone gamma-carboxyglutamic acid-containing protein (OC) |
| BMD | bone mineral density |
| CASP | caspase |
| c-FOS | cellular protooncogene |
| COHA | cobalt-substitute hydroxyapatitie |
| COL1A1 | Collagen alpha-1 (I) chain precursor |
| CSF1 | Colony stimulating factor 1 |
| CSCM | 30% calvaria-derived stromal cell conditioned media |
| DEPC | sterile filter water treated with diethyl pyrocarbonate |
| DMP1 | dentin matrix acidic phosphoprotein 1 |
| ECM | extracellular matrix |
| FBS | Fetal Bovine Serum |
| FTIR-ATR | Fourier Transform Infrared-Attenuated Total Reflectance |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| IL | interleukin |
| IONs | iron oxide nanoparticles |
| ITGAV | Integrin Subunit AlphaV |
| iNOS | inducible nitric oxide synthase |
| INT | integrin |
| MEMα | Minimum Essential Medium α |
| miRNA | microRNA |
| MF | magnetic field |
| MMP | matrix metalloproteinase |
| MNPs | magnetic nanoparticles |
| OC | osteocalcin (BGLAP) |
| OPN | osteopontin |
| OS | osteoporosis |
| p21 | cyclin-dependent kinase inhibitor 1 |
| p53 | tumor suppressor factor |
| PMMA | polymethylcrylate |
| PU.1 | protein in human encoded by the SPI1 gene |
| RANKL | Receptor activator of NFκβ ligand |
| RUNX2 | Runt-related transcription factor 2 |
| SAED | selected area (electron) diffraction |
| SEM | scanning electron microscopy |
| SMF | static magnetic field |
| TEM | transmission electron microscopy |
| TNFα | tumor necrosis factor α |
| TGFβ | transforming growth factor β |
References
- Akkawi, I.; Zmerly, H. Osteoporosis: Current Concepts. Joints 2018, 6, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef]
- Teng, G.G.; Curtis Effrey, R.; Saag, K.G. Mortality and osteoporotic fractures: Is the link causal, and is it modifiable? Clin. Exp. Rheumatol. 2008, 26, S125–S137. [Google Scholar] [PubMed]
- Knight, M.N.; Hankenson, K.D. Mesenchymal Stem Cells in Bone Regeneration. Adv. Wound Care 2013, 2, 306–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caetano-Lopes, J.; Canhão, H.; Fonseca, J.E. Osteoblasts and bone formation. Acta Reumatol. Port. 2007, 32, 103–110. [Google Scholar] [PubMed]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Hou, W.; Zhou, Y. Osteopontin in Bone Metabolism and Bone Diseases. Med. Sci. Monit. 2020, 26, e919159-1. [Google Scholar] [CrossRef]
- Charles, J.; Aliprantis, A.O. Osteoclasts: More than ‘bone eaters’. Trends Mol. Med. 2014, 20, 449–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerlov, C.; Graf, T. PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 1998, 12, 2403–2412. [Google Scholar] [CrossRef] [Green Version]
- Giustini, A.J.; Petryk, A.A.; Cassim, S.M.; Tate, J.A.; Baker, I.; Hoopes, P.J. MAGNETIC NANOPARTICLE HYPERTHERMIA IN CANCER TREATMENT. Nano Life 2010, 01, 17–32. [Google Scholar] [CrossRef]
- Frazer, R.Q.; Byron, R.T.; Osborne, P.B.; West, K.P. PMMA: An Essential Material in Medicine and Dentistry. J. Autom. Inf. Sci. 2005, 15, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Yip, K.W.; Mao, X.; Au, P.B.; Hedley, D.; Chow, S.; Dalili, S.; Mocanu, J.D.; Bastianutto, C.; Schimmer, A.; Liu, F.-F. Benzethonium Chloride: A Novel Anticancer Agent Identified by Using a Cell-Based Small-Molecule Screen. Clin. Cancer Res. 2006, 12, 5557–5569. [Google Scholar] [CrossRef] [Green Version]
- Shima, H.; Kawai, T.; Matsushima, Y.; Unuma, H.; Kawamura, K.; Li, Z.; Kawashita, M. Magnetite fine particles highly loaded PMMA microspheres for hyperthermia of deep-seated cancer. J. Ceram. Soc. Jpn. 2013, 121, 802–806. [Google Scholar] [CrossRef] [Green Version]
- Kulpa-Greszta, M.; Pązik, R.; Kłoda, P.; Tomaszewska, A.; Zachanowicz, E.; Pałka, K.; Ginalska, G.; Belcarz, A. Efficient non-contact heat generation on flexible, ternary hydroxyapatite/curdlan/nanomagnetite hybrids for temperature controlled processes. Mater. Sci. Eng. C 2021, 118, 111360. [Google Scholar] [CrossRef]
- Zachanowicz, E.; Zięcina, A.; Mikołajczyk, P.A.; Rogacki, K.; Małecka, M.; Marycz, K.; Marędziak, M.; Poźniak, B.; Nowakowska, M.; Tikhomirov, M.; et al. Cytotoxic Effects of Co1−xMnxFe2O4 Ferrite Nanoparticles Synthesized under Non-Hydrolytic Conditions (Bradley’s Reaction)—In Vitro. Eur. J. Inorg. Chem. 2016, 2016, 5315–5323. [Google Scholar] [CrossRef]
- Zachanowicz, E.; Pigłowski, J.; Grzymajło, M.; Poźniak, B.; Tikhomirov, M.; Pierunek, N.; Śniadecki, Z.; Idzikowski, B.; Marycz, K.; Maredziak, M.; et al. Efficient synthesis of PMMA@Co0.5Ni0.5Fe2O4 organic-inorganic hybrids containing hyamine 1622—Physicochemical properties, cytotoxic assessment and antimicrobial activity. Mater. Sci. Eng. C 2018, 90, 248–256. [Google Scholar] [CrossRef]
- Zachanowicz, E.; Kulpa-Greszta, M.; Tomaszewska, A.; Gazińska, M.; Marędziak, M.; Marycz, K.; Pązik, R. Multifunctional Properties of Binary Polyrhodanine Manganese Ferrite Nanohybrids—from the Energy Converters to Biological Activity. Polymer 2020, 12, 2934. [Google Scholar] [CrossRef]
- ImageJ, v.1.46. Available online: https://imagej.nih.gov/ij/ (accessed on 1 September 2021).
- Amano, S.; Sekine, K.; Bonewald, L.F.; Ohmori, Y. A novel osteoclast precursor cell line, 4B12, recapitulates the features of primary osteoclast differentiation and function: Enhanced transfection efficiency before and after differentiation. J. Cell. Physiol. 2009, 221, 40–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gen5, Version 2.0, Data Analysis Software. Available online: https://www.labbulletin.com/articles/gen5-version-2-0-data-analysis-software-now-available-from-biotek (accessed on 1 September 2021).
- Marycz, K.; Sobierajska, P.; Roecken, M.; Kornicka-Garbowska, K.; Kępska, M.; Idczak, R.; Nedelec, J.-M.; Wiglusz, R.J. Iron oxides nanoparticles (IOs) exposed to magnetic field promote expression of osteogenic markers in osteoblasts through integrin alpha-3 (INTa-3) activation, inhibits osteoclasts activity and exerts anti-inflammatory action. J. Nanobiotechnol. 2020, 18, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chomczynski, P. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate–Phenol–Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, 2nd ed.; Wiley-VCH: Weinheim, Germany, 1974; p. 992. Available online: https://ui.adsabs.harvard.edu/abs/1974xdpf.book.....K/abstract (accessed on 1 September 2021).
- Fan, W.; Crawford, R.; Xiao, Y. Enhancing in vivo vascularized bone formation by cobalt chloride-treated bone marrow stromal cells in a tissue engineered periosteum model. Biomaterials 2010, 31, 3580–3589. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Yang, Y.; Deng, Y. Dual therapeutic cobalt-incorporated bioceramics accelerate bone tissue regeneration. Mater. Sci. Eng. C 2019, 99, 770–782. [Google Scholar] [CrossRef]
- Phakatkar, A.H.; Shirdar, M.R.; Qi, M.-L.; Taheri, M.M.; Narayanan, S.; Foroozan, T.; Sharifi-Asl, S.; Huang, Z.; Agrawal, M.; Lu, Y.-P.; et al. Novel PMMA bone cement nanocomposites containing magnesium phosphate nanosheets and hydroxyapatite nanofibers. Mater. Sci. Eng. C 2020, 109, 110497. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, N.M.; Murugesan, S. Preparation and characterization of an iron oxide-hydroxyapatite nanocomposite for potential bone cancer therapy. Int. J. Nanomed. 2015, 10, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Vlad, M.D.; del Valle, L.J.; Barracó, M.; Torres, R.; López, J.; Fernández, E. Iron Oxide Nanoparticles Significantly Enhances the Injectability of Apatitic Bone Cement for Vertebroplasty. Spine 2008, 33, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhou, Y.; Zhao, Y.; Xu, Y.; Zhang, F.; Gu, N.; Ma, J.; Reynolds, M.A.; Xia, Y.; Xu, H.H. Enhanced bone regeneration and visual monitoring via superparamagnetic iron oxide nanoparticle scaffold in rats. J. Tissue Eng. Regen. Med. 2018, 12, e2085–e2098. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.; Di Silvio, L.; Harper, E.; Bonfield, W. Increasing hydroxyapatite incorporation into poly(methylmethacrylate) cement increases osteoblast adhesion and response. Biomaterials 2002, 23, 569–576. [Google Scholar] [CrossRef]
- Pazik, R.; Piasecka, E.; Małecka, M.; Kessler, V.; Idzikowski, B.; Sniadecki, Z.; Wiglusz, R.J. Facile non-hydrolytic synthesis of highly water dispersible, surfactant free nanoparticles of synthetic MFe2O4 (M–Mn2+, Fe2+, Co2+, Ni2+) ferrite spinel by a modified Bradley reaction. RSC Adv. 2013, 3, 12230–12243. [Google Scholar] [CrossRef]
- Alicka, M.; Major, P.; Wysocki, M.; Marycz, K. Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Type 2 Diabetes Show Reduced “Stemness” through an Altered Secretome Profile, Impaired Anti-Oxidative Protection, and Mitochondrial Dynamics Deterioration. J. Clin. Med. 2019, 8, 765. [Google Scholar] [CrossRef] [Green Version]












| Gene | Forward (5′→3′) | Reverse (3′→5′) | Length of Amplicon |
|---|---|---|---|
| P21 | TGTTCCACACAGGAGCAAAG | AACACGCTCCCAGACGTAGT | 175 |
| P53 | AGTCACAGCACATGACGGAGG | GGAGTCTTCCAGTGTGATGATGG | 287 |
| CASP9 | CCGGTGGACATTGGTTCTGG | GCCATCTCCATCAAAGCCGT | 278 |
| BAD | ACATTCATCAGCAGGGACGG | ATCCCTTCATCCTCCTCGGT | 115 |
| BAX | AGGACGCATCCACCAAGAAGC | GGTTCTGATCAGCTCGGGCA | 251 |
| BCL2 | GGATCCAGGATAACGGAGGC | ATGCACCCAGAGTGATGCAG | 141 |
| RUNX2 | TCCGAAATGCCTCTGCTGTT | GCCACTTGGGGAGGATTTGT | 130 |
| RANKL | TTAAGCCAGTGCTTCACGGG | ACGTAGACCACGATGATGTCGC | 493 |
| OPG | TGGCACACAGTGATGAATGCG | GCTGGAAAGTTTGCTCTTGCG | 149 |
| ALP | TTCATAAGCAGGCGGGGGAG | TGAGATTCGTCCCTCGCTGG | 198 |
| COL1A1 | CCAGCCGCAAAGAGTCTACA | CAGGTTTCCACGTCTCACCA | 175 |
| OPN | AGACCATGCAGAGAGCGAG | GCCCTTTCCGTTGTTGTCCT | 340 |
| BGLAP2 | CTCCTGAGAGTCTGACAAAGCCTT | GCTGTGACATCCATTACTTGC | 320 |
| DMP1 | CCCAGAGGCACAGGCAAATA | TCCTCCCCAATGTCCTTCTT | 211 |
| MMP9 | TTGCCCCTACTGGAAGGTATTAT | GAGAATCTCTGAGCAATCCTTGA | 172 |
| PU.1 | GAGAAGCTGATGGCTTGGAG | TTGTGCTTGGACGAGAACTG | 175 |
| ITGAV | ACAATGTAAGCCCAGTTGTGTCT | TTTGTAAGGCCACTGGAGATTTA | 236 |
| C-FOS | CCAGTCAAGAGCATCAGCAA | TAAGTAGTGCAGCCCGGAGT | 248 |
| INTa1 | CACCTTTCAAACTGAGCCCGCCA | GCTGCCCAGCGATGTAGAGCACAT | 110 |
| INTa3 | TGGGCAAGTGCTATGTGCGTGGCA | TCTGGGTGAAGCCGCCGCTGGT | 147 |
| INTa6 | CTGGCTTCCTCGTTTGGCTATG | TGCCTTGCTGGTTAATGTAGACGT | 145 |
| INTb1 | TCTCACCAAAGTAGAAAGCAGGGA | ACGATAGCTTCATTGTTGCCATTC | 138 |
| IL6 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA | 141 |
| TGFβ1 | GGAGAGCCCTGGATACCAAC | CAACCCAGGTCCTTCCTAAA | 171 |
| TNFα | GAACTGGCAGAAGAGGCACT | AGGGTCTGGGCCATAGAACT | 203 |
| miR-7a-5p | TGGAAGACTAGTGATTTTGTTGT | * | |
| miR-17-5p | CAAAGTGCTTACAGTGCAGGTAG | * | |
| miR-145-5p | GTCCAGTTTTCCCAGGAATCCCT | * | |
| miR-21-5p | TAGCTTATCAGACTGATGTTGA | * | |
| miR-124-3p | TAAGGCACGCGGTGAATGCCAA | * | |
| miR-203a | GUGAAAUGUUUAGGACCACUAG | * | |
| miR-223a | TGTCAGTTTGTCAAATACCCCA | * | |
| GAPDH | TGCACCACCAACTGCTTAG | GGATGCAGGGATGATGTTC | 177 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marycz, K.; Turlej, E.; Kornicka-Garbowska, K.; Zachanowicz, E.; Tomaszewska, A.; Kulpa-Greszta, M.; Pązik, R. Co0.5Mn0.5Fe2O4@PMMA Nanoparticles Promotes Preosteoblast Differentiation through Activation of OPN-BGLAP2-DMP1 Axis and Modulates Osteoclastogenesis under Magnetic Field Conditions. Materials 2021, 14, 5010. https://doi.org/10.3390/ma14175010
Marycz K, Turlej E, Kornicka-Garbowska K, Zachanowicz E, Tomaszewska A, Kulpa-Greszta M, Pązik R. Co0.5Mn0.5Fe2O4@PMMA Nanoparticles Promotes Preosteoblast Differentiation through Activation of OPN-BGLAP2-DMP1 Axis and Modulates Osteoclastogenesis under Magnetic Field Conditions. Materials. 2021; 14(17):5010. https://doi.org/10.3390/ma14175010
Chicago/Turabian StyleMarycz, Krzysztof, Eliza Turlej, Katarzyna Kornicka-Garbowska, Emilia Zachanowicz, Anna Tomaszewska, Magdalena Kulpa-Greszta, and Robert Pązik. 2021. "Co0.5Mn0.5Fe2O4@PMMA Nanoparticles Promotes Preosteoblast Differentiation through Activation of OPN-BGLAP2-DMP1 Axis and Modulates Osteoclastogenesis under Magnetic Field Conditions" Materials 14, no. 17: 5010. https://doi.org/10.3390/ma14175010