“Success Depends on Your Backbone”—About the Use of Polymers as Essential Materials Forming Orodispersible Films
Abstract
:1. Introduction
2. Hydrophilic Polymeric Materials
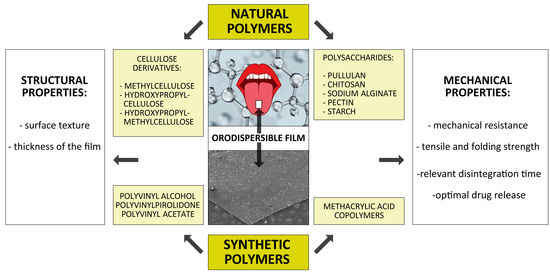
2.1. Natural Polymers
2.1.1. Cellulose and Cellulose Derivatives
2.1.2. Starch and Starch Derivatives
2.1.3. Sodium Alginate and Chitosan
2.2. Synthetic Polymeric Materials
2.2.1. Polyvinyl Alcohol (PVA)
2.2.2. Polyvinyl Pyrrolidone (Povidone, PVP)
2.2.3. Polyethylene Oxide (PEO)
3. Hydrophobic Polymeric Materials
4. Overview of ODF Formulations Available on the Pharmaceutical Market
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Routes of Administration of Drug Dosage Forms According to FDA. Available online: https://www.fda.gov/drugs/data-standards-manual-monographs/route-administration (accessed on 10 March 2021).
- Van Riet-Nales, D.A.; Ferreira, J.A.; Schobben, A.F.A.M.; de Neef, B.J.; Egberts, T.C.; Rademaker, C.M. Methods of administering oral formulations and child acceptability. Int. J. Pharm. 2015, 491, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Preis, M. Orally disintegrating films and mini-tablets—Innovative dosage forms of choice for pediatric use. AAPS PharmSciTech 2015, 16, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Alany, R. Oral dosage forms and drug delivery systems: Tablets, oral films, liquid dosage forms, oral bioavailability enhancement. Pharm. Dev. Technol. 2017, 22, 137. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, E.M.; Breitenbach, A.; Breitkreutz, J. Advances in orodispersible films for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Mahboob, T.R.; Jamshaid, M.; Bashir, I.; Zulfiqar, S. Oral films: A comprehensive review. Int. Cur. Pharm. J. 2016, 5, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.F.; Silva, C.; Coelho, J.F.; Simões, S. Oral films: Current status and future perspectives I—Galenical development and quality attributes. J. Control. Release 2015, 206, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cilurzo, F.; Musazzi, U.M.; Franze, S.; Selmin, F.; Minghetti, P. Orodispersible dosage forms: Biopharmaceutical improvements and regulatory requirements. Drug Discov. Today 2018, 23, 251–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irfan, M.; Rabel, S.; Bukhtar, Q.; Qadir, M.I.; Jabeen, F.; Khan, A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm. J. 2016, 24, 537–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpa, M.; Stegemann, S.; Hsiao, W.K.; Pichler, H.; Gaisford, S.; Bresciani, M.; Paudel, A.; Orlu, M. Orodispersible films: Towards drug delivery in special populations. Int. J. Pharm. 2017, 523, 327–335. [Google Scholar] [CrossRef] [PubMed]
- The European Pharmacopoeia, 10th ed.; Council of Europe: Strasburg, France, 2019.
- FDA; CDER. Guidance for Industry—Orally Disintegrating Tablets. 2008. Available online: https://www.fda.gov/downloads/Drugs/Guidances/ucm070578.pdf (accessed on 15 April 2021).
- The United States Pharmacopeia and National Formulary (USP41-NF 36); Pharmacopeia Convention: Rockville, MD, USA, 2018; Volume 2.
- Cilurzo, F.; Cupone, I.E.; Minghetti, P.; Selmin, F.; Montanari, L. Fast dissolving films made of maltodextrin. Eur. J. Pharm. Biopharm. 2008, 70, 895–900. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Mane, M.; Ghadge, D. Exploration of different polymers for use in the formulation of oral fast dissolving strips. J. Cur. Pharm. Res. 2010, 2, 33–35. [Google Scholar]
- Liew, K.B.; Tan, Y.T.F.; Peh, K.-K. Effect of polymer, plasticizer and filler on orally disintegrating films. Drug Dev. Ind. Pharm. 2014, 40, 110–119. [Google Scholar] [CrossRef]
- Amelian, A.; Szymańska, E.; Winnicka, K. Formulation and characterization of loratadine containing orodispersible lyophilizates and films. Acta Pol. Pharm. Drug Res. 2017, 74, 1533–1541. [Google Scholar]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK; Chicago, IL, USA; Washington, DC, USA, 2009. [Google Scholar]
- Pathare, Y.S.; Hastak, V.S.; Bajaj, A.N. Polymers used for fast disintegrating oral films: A review. Int. J. Pharm. Sci. Rev. Res. 2013, 21, 169–178. [Google Scholar]
- Qui, Y.; Zhang, G.G.Z.; Mantri, R.V.; Chen, Y.; Yu, L. Developing Solid Oral Dosage Forms. Pharmaceutical Theory and Practice, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- FDA Inactive Ingredients Database. Available online: https://search.fda.gov/search?utf8=%E2%9C%93&affiliate=fda1&query=ethylcellulose&commit=Search (accessed on 10 March 2021).
- Safety & Toxicity of Excipients for Paediatrics, STEP Database. Available online: http://www.eupfi.org/step-database-info/ (accessed on 10 March 2021).
- Mašková, E.; Kubová, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlídalová, E.; Turánek, J.; Mašek, J. Hypromellose—A traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J. Control. Release 2020, 324, 695–727. [Google Scholar] [CrossRef]
- Methocel. Available online: https://www.pharma.dupont.com//pharmaceutical-brands/methocel.html (accessed on 21 April 2021).
- Pharmacoat, Metolose. Available online: https://www.metolose.jp/en/pharmaceutical/ (accessed on 21 April 2021).
- Benecel. Available online: https://www.ashland.com/industries/pharmaceutical/oral-solid-dose/benecel-methylcellulose-and-hypromellose (accessed on 21 April 2021).
- Gustafsson, C.; Bonferoni, M.C.; Caramella, C.; Lennholm, H.; Nystrom, C. Characterisation of particle properties and compaction behaviour of hydroxypropylmethylcellulose with different degrees of methoxy/hydroxypropyl substitution. Eur. J. Pharm. Sci. 1999, 9, 171–184. [Google Scholar] [CrossRef]
- Affinisol. Available online: https://www.pharma.dupont.com/pharmaceutical-brands/affinisol.html (accessed on 21 April 2021).
- Repka, M.A.; Gutta, K.; Prodduturi, S.; Munjal, M.; Stodghill, S.P. Characterization of cellulosic hot-melt extruded films containing lidocaine. Eur. J. Pharm. Biopharm. 2005, 59, 189–196. [Google Scholar] [CrossRef]
- Mahesh, A.; Sadanandam, M. Development of taste masked fast disintegrating films of levocetirizine dihydrochloride for oral use. Curr. Drug Deliv. 2010, 7, 21–27. [Google Scholar] [CrossRef]
- Sharma, R.; Gohel, M.; Soniwala, M. Development of taste masked film of valdecoxib for oral use. Indian J. Pharm. Sci. 2007, 69, 318–320. [Google Scholar] [CrossRef] [Green Version]
- Centkowska, K.; Ławrecka, E.; Sznitowska, M. Technology of orodispersible polymer films with micronized loratadine—influence of different drug loadings on film properties. Pharmaceutics 2020, 12, 250. [Google Scholar] [CrossRef] [Green Version]
- Woertz, C.; Kleinebudde, P. Development of orodispersible polymer films containing poorly water soluble active pharmaceutical ingredients with focus on different drug loadings and storage stability. Int. J. Pharm. 2015, 493, 134–145. [Google Scholar] [CrossRef]
- Maddel, S.; Nalluri, B.N. Development of zolmitriptan mouth dissolving films: Formulation variables, mechanical properties, and in vitro drug release studies. Asian J. Pharm. Clin. Res. 2019, 12, 4. [Google Scholar]
- Ma, Y.; Guan, R.; Gao, S.; Song, W.; Yang, Y.; Liu, H. Designing orodispersible films containing everolimus for enhanced compliance and bioavailability. Expert Opin. Drug. Deliv. 2020, 17, 1499–1508. [Google Scholar] [CrossRef]
- El-Bary, A.A.; Al Sharabi, I.; Saeed-Haza’a, B. Effect of casting solvent, film-forming agent and solubilizer on orodispersible films of a polymorphic poorly soluble drug: An in vitro/in silico study. Drug. Dev. Ind. Pharm. 2019, 45, 1751–1769. [Google Scholar] [CrossRef] [PubMed]
- Brniak, W.; Maślak, E.; Jachowicz, R. Orodispersible films and tablets with prednisolone microparticles. Eur. J. Pharm. Sci. 2015, 75, 81–90. [Google Scholar] [CrossRef]
- Senta-Loys, Z.; Bourgeois, S.; Valour, J.-P.; Briancon, S.; Fessi, H. Orodispersible films based on amorphous solid dispersions of tetrabenazine. Int. J. Pharm. 2017, 25, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Senta-Loys, Z.; Bourgeois, S.; Pailler-Mattei, C.; Agusti, G.; Briancon, S.; Fessi, H. Formulation of orodspersible films for paediatric therapy: Investigation of feasibility and stability for tetrabenazine as drug model. J. Pharm. Pharmacol. 2017, 69, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Panraksa, P.; Udomsom, S.; Rachtanapun, P.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. Hydroxypropyl methylcellulose E15: A hydrophilic polymer for fabrication of orodispersible film using syringe extrusion 3D printer. Polymers 2020, 12, 2666. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.A.; Karpagam, S. Cellulose orodispersible films of donepezil: Film characterization and drug release. Pharm. Chem. J. 2017, 51, 707–715. [Google Scholar] [CrossRef]
- Visser, J.C.; Weggemans, O.A.F.; Boosman, R.J.; Loos, U.K.; Frijlink, H.W.; Woerdenbag, H.J. Increased drug load and polymer compatibility of bilayered orodispersible films. Eur. J. Pharm. Sci. 2017, 30, 183–190. [Google Scholar] [CrossRef]
- Dinge, A.; Nagarsenker, M. Formulation and evaluation of fast dissolving films for delivery of triclosan to the oral cavity. AAPS PharmSciTech 2008, 9, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, R.; Gupta, A.K.; Parashar, A.K. Formulation and evaluation of fast dissolving films of granisetron hydrochloride. Int. Res. J. Pharm. 2015, 6, 724–728. [Google Scholar] [CrossRef]
- Dixit, A.S.; Kulkarni, P.K. Fast disintegrating films containing anastrazole as dosage form for dysphagia patients. Arch. Pharm. Res. 2012, 35, 2171–2182. [Google Scholar] [CrossRef]
- Tian, Y.; Bhide, Y.C.; Woerdenbag, H.J.; Huckriede, A.L.; Frijlink, H.W.; Hinrichs, W.L.J.; Visser, J.C. Development of an orodispersible film containing stabilized influenza vaccine. Pharmaceutics 2020, 12, 245. [Google Scholar] [CrossRef] [Green Version]
- Edinger, M.; Bar-Shalom, D.; Sandler, N.; Rantanen, J.; Genina, N. QR encoded smart oral dosage forms by inject printing. Int. J. Pharm. 2018, 536, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Mushtaque, M.; Muhammad, I.N.; Hassan, S.M.F.; Ali, A.; Masood, R. Development and pharmaceutical evaluation of oral fast dissolving thin film of escitalopram: A patient friendly dosage form. Pak. J. Pharm. Sci. 2020, 33, 183–189. [Google Scholar]
- Yan, T.-T.; Lv, Z.-F.; Tian, P.; Lin, M.-M.; Lin, W.; Huang, S.-Y.; Chen, Y.-Z. Semi-solid extrusion 3D printing ODFs: An individual drug delivery system for small scale pharmacy. Drug Dev. Ind. Pharm. 2020, 46, 531–538. [Google Scholar] [CrossRef]
- Serrano, D.R.; Fernandez-Garcia, R.; Mele, M.; Healy, A.M.; Lalatsa, A. Designing fast-dissolving orodispersible films of amphotericin B for oropharyngeal candidiasis. Pharmaceutics 2019, 11, 369. [Google Scholar] [CrossRef] [Green Version]
- Mateescu, M.A.; Ispas-Szabo, P.; Assaad, E. Starch and derivatives as pharmaceutical excipients: From nature to pharmacy. In Controlled Drug Delivery. The Role of Self-Assembling Multitask Excipients, 1st ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 21–84. [Google Scholar]
- Bertoft, E. Understanding starch structure: Recent progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef]
- Lycoat. Available online: https://www.roquette.com/pharma-and-nutraceuticals-hydroxypropyl-pea-starch-lycoat (accessed on 21 April 2021).
- Kathpalia, H.; Patil, A. Formulation and evaluation of orally disintegrating films of levocetirizine dihydrochloride. Ind. J. Pharm. Sci. 2017, 79, 204–211. [Google Scholar] [CrossRef]
- Kunte, S.; Tandale, P. Fast dissolving strips: A novel approach for the delivery of verapamil. J. Pharm. Bioallied. Sci. 2010, 2, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, U.M.; Dolci, L.S.; Albertini, B.; Passerini, N.; Cilurzo, F. A new melatonin oral delivery platform based on orodispersible films containing solid lipid microparticles. Int. J. Pharm. 2019, 559, 280–288. [Google Scholar] [CrossRef] [Green Version]
- Pechova, V.; Gajdziok, J.; Muselik, J.; Vetchy, D. Development of orodispersible films containing benzydamine hydrochloride using modified solvent casting method. AAPS PharmSciTech 2018, 19, 2509–2518. [Google Scholar] [CrossRef]
- Cupone, I.E.; Dellera, E.; Marra, F.; Giori, A.M. Development and characterization of an orodispersible film for vitamin D3 supplementation. Molecules 2020, 25, 5851. [Google Scholar] [CrossRef]
- Jaipakdeeb, N.; Limpongsaa, E. Physical modification of Thai rice starch and its application as orodispersible film former. Carbohydr. Polym. 2020, 239. [Google Scholar] [CrossRef]
- Kumar, D.; Saini, N.; Pandit, V.; Ali, S. An insight to pullulan: A biopolymer in pharmaceutical approaches. Int. J. Basic Appl. Sci. 2012, 1, 202–219. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiang, Y.; Sheng, R.; Tomas, H.; Rodrigues, S.; Gu, Z.; Zhang, H.; Gong, Q.; Luo, K. Polisaccharide-based nanomedicines for cancer immunotherapy: A review. Bioact. Mater. 2021, 6, 3358–3382. [Google Scholar] [CrossRef]
- Chachlioutaki, K.; Tzimtzimis, E.K.; Tzetzis, D.; Chang, M.-W.; Ahmad, Z.; Karavasili, C.; Fatouros, D. Electrospun orodispersible film of isoniazid for pediatric tuberculosis treatment. Pharmaceutics 2020, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Shia, L.-L.; Xua, W.-J.; Caoa, Q.-R.; Cui, J. Preparation, characterization and in vitro evaluation of a polyvinyl alcohol/sodium alginate based orodispersible film containing sildenafil citrate. Pharmazie 2014, 69, 327–334. [Google Scholar]
- Koteswari, P.; Sravanthi, G.P.; Mounika, M.; Rafi, S.K.M.; Nirosha, K. Formulation development and evaluation of zolmitriptan oral soluble films using 2 factorial design. Int. J. Pharm. Investig. 2016, 6, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.A.; Karpagam, S. In vitro and in vivo evaluation of oral disintegrating nanofiber and thin-film contains hyperbranched chitosan/donepezil for active drug delivery. J. Pol. Envir. 2021, 29, 922–936. [Google Scholar]
- Qin, Z.-Y.; Jia, X.-W.; Liu, Q.; Kong, B.-H.; Wang, H. Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, Y.; Maqbool, M.; Hussain, T.; Yousaf, A.M.; Khan, I.U.; Mahmood, T.; Jamshaid, M. Natural and semisynthetic polymers blended orodispersible films of citalopram. Nat. Prod. Res. 2020, 32, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Tosif, M.M.; Najda, A.; Bains, A.; Kaushnik, R.; Dhull, S.B.; Chawla, P.; Walasek-Janusz, M. A comprehensive review on plant-derived mucilage: Characterization, functional properties, applications, and its utilization for nanocarrier fabrication. Polymers 2021, 13, 1066. [Google Scholar] [CrossRef] [PubMed]
- Muppalaneni, S. Polyvinyl alcohol in medicine and pharmacy: A perspective. J. Develop. Drugs 2013, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Mashru, R.C.; Sutariya, V.B.; Sankalia, M.G.; Parikh, P.P. Development and evaluation of fast-dissolving film of salbutamol sulphate. Drug Dev. Ind. Pharm. 2005, 31, 25–34. [Google Scholar] [CrossRef]
- Zaman, M.; Hassan, R.; Razzaq, S.; Mahmood, A.; Wahab, M.; Raja, M.A.G.; Qaisar, A.A.; Majeed, A.; Hanif, M.; Tahir, R.A. Fabrication of polyvinyl alcohol based fast dissolving oral strips of sumatriptan succinate and metoclopramide HCL. Sci. Prog. 2020, 103, 1–21. [Google Scholar] [CrossRef]
- Panraksa, P.; Tipduangta, P.; Jantanasakulwong, K.; Jantrawut, P. Formulation of orally disintegrating films as an amorphous solid solution of a poorly water-soluble drug. Membranes 2020, 10, 376. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M. Poly(vinylpyrrolidone)—A versatile polymer for biomedical and beyond medical applications. Polym. Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Kumar, P.; Phani, A.R.; Prasad, R.G.S.V.; Sanganal, J.S.; Manali, N.; Gupta, R.; Rashmi, N.; Prabhakara, G.S.; Salins, P.; Sandeep, K.; et al. Polyvinylpyrrolidone oral films of enrofloxacin: Film characterization and drug release. Int. J. Pharm. 2015, 471, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.R.; Chaudhary, S.A.; Mehta, T.A. Polyox (polyethylene oxide) multifunctional polymer in novel drug delivery system. Int. J. Pharm. Sci. Drug Res. 2014, 6, 95–101. [Google Scholar]
- Cho, H.-W.; Baek, S.-H.; Lee, B.-J.; Jin, H.-E. Orodispersible polymer films with the poorly water-soluble drug, olanzapine: Hit-melt pneumatic extrusion for single process 3D printing. Pharmaceutics 2020, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.F.; Silva, B.M.A.; Silva, C.; Coelho, J.F.J.; Simoes, S. Hydrophobic polymers for orodispersible films: A quality by design approach. Expert Opin. Drug Deliv. 2016, 13, 1357–1374. [Google Scholar] [CrossRef] [PubMed]
- Mussazi, U.M.; Selmin, F.; Franze, S.; Gennari, C.G.M.; Rocco, P.; Minghetti, P.; Cilurzo, F. Poly(methyl methacrylate) salt as film forming material to design orodispersible films. Eur. J. Pharm. Sci. 2018, 115, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niquitin Mint Oral Strips. Available online: https://www.hpra.ie/img/uploaded/swedocuments/LicenseSPC_PA0678-071-018_27112015134038.pdf (accessed on 10 March 2021).
- Benadryl Allergy Quick Dissolve Strip. Available online: https://www.drugs.com/otc/106077/benadryl-allergy-quick-dissolve.htmL (accessed on 10 March 2021).
- Chloraseptic Sore Throat Relief Strips. Available online: https://www.robertfreshmarket.com/shop/chloraseptic_sore_throat_relief_strips_cherry/p/2432811 (accessed on 10 March 2021).
- D-Fusion Film 800 UI. Available online: https://www.newpharma.de/d-fusion/824326/d-fusion-film-balans-800-ui-ie-28.html (accessed on 10 March 2021).
- Exservan Oral Film. Available online: https://www.rxlist.com/exservan-drug.html (accessed on 10 March 2021).
- Gas-X Thin Strips. Available online: https://www.walmart.com/ip/Gas-X-Thin-Strips-Extra-Strength-Peppermint-18-Each-Pack-of-3/357662639 (accessed on 10 March 2021).
- IvyFilm, IvyFilm Kiddies. Available online: http://lamar.co.za/sites/default/files/files/IvyFilm%20PIL%20L10024_14A_PROOF.pdf (accessed on 10 March 2021).
- Listerine PocketPaks Oral Care Strips. Available online: https://www.listerine.com/on-the-go-oral-health/listerine-pocketpaks-cool-mint (accessed on 10 March 2021).
- Pedia-Lax Quick Dissolve Strip. Available online: https://www.drugs.com/sfx/pedia (accessed on 10 March 2021).
- Sudafed PE. Available online: https://www.webmd.com/drugs/2/drug-144195/sudafed-pe-quick-dissolve-oral/details (accessed on 10 March 2021).
- SildeHEXAL SF Orodispersible Film. Available online: https://downloads.dokteronline.com/leaflets/en/patient_information_leaflet-2646-sildehexal-uk.pdf-1510756508.pdf (accessed on 10 March 2021).
- Sildenafil IBSA Orodispersible Film. Available online: https://www.drugs.com/uk/sildenafil-ibsa-100-mg-orodispersible-film-leaflet.html (accessed on 10 March 2021).
- Tusheel. Available online: https://www.heel.es/es/tusheel.html?logedin=5B4E1F806FDB5A5C1981C9D79191E9C0 (accessed on 10 March 2021).
- Setofilm Data. Available online: https://www.medicines.org.uk/emc/product/9595/smpc#gref (accessed on 10 March 2021).
- Sympazan Oral Film. Available online: https://www.sympazan.com/about-sympazan-clobazam.html (accessed on 10 March 2021).
- Zentrip. Available online: https://www.drugs.com/otc/116987/zentrip.html (accessed on 10 March 2021).
- Zuplenz. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022524s000lbl.pdf (accessed on 10 March 2021).













| Cellulose Derivative | Manufacturing Metod | Characteristic |
|---|---|---|
| Methylcellulose (MC) | Produced in the reaction of methyl chloride and a cellulose activated by mercerizing the cellulose with aqueous alkali in the presence of a C2-C3 alkyl chloride as a reaction medium at a temperature from 65 °C to 90 °C and at pressure from 3 to 15 bar. | Methyl ether of cellulose, in which the content of methoxyl groups is 27–32%. It takes the form of white, light-yellow, or light-gray powder or granulate. It does not dissolve in hot water, but swells in cold water and forms dispersions. The degree of substitution (DS) influences the solubility and viscosity of the polymer. The viscosity of the 2% (w/w) aqueous solution ranges from 5 to 75,000 mPa⋅s. DS is determined as the average level of substituted hydroxyl groups per glucose unit in cellulose molecule. Its value is in the range from 0 to 3. |
| Carboxymethyl- cellulose sodium salt (NaCMC) | Synthesized by the alkali-catalyzed reaction of cellulose with monochloroacetic acid or its sodium salt under alkaline conditions in the presence of an organic solvent. Hydroxyl groups replaced by sodium carboxymethyl groups in C2, C3 and C6 of glucose, the substitution being slightly predominant at the C2 position. | Semisynthetic, linear, anionic derivative of cellulose, known as crosscarmellose sodium. White, odorless powder of molecular weight 90,000–700,000. Readily dispersed in water, forming a transparent or colloidal solution. One percent viscosity of the aqueous solution ranges from 5–13,000 mPa⋅s. NaCMC plays a significant role as a thickener and is suitable for applications requiring a rapidly soluble base. DS value of NaCMC has a significant effect on the properties of membrane-forming solutions because higher DS values are closely associated with reduced inter-chain interactions considering the increased substitution of hydroxyl sites. NaCMC has been found to be a valuable excipient for the development of ODFs with good transparency and capacity for a broad array of drugs. NaCMC-based films are also characterized by the ability to carry a broad spectrum of DS and good compatibility with starch to form single-phase polymeric matrix films with enhanced mechanical or barrier characteristics. |
| Hydroxyethyl- cellulose (HEC) | Obtained by introduction of ammonia-activated cellulose into water sodium hydroxide solution, which is responsible for its swelling, then degassed and mixed with an appropriate amount of III-row butanol. The processes take place in the presence of nitrogen and the introduction of ethylene oxide. The whole is neutralized by acetic acid and dried. | Fully fragranceless, flavorless, and non-toxic white to pale-yellow powder that is easily dissolved in hot and cold water and insoluble in organic solvents. After dissolution in water it creates a transparent, viscous solution. There are varieties available with different degrees of substitution, with 2% water solutions varying in viscosity from 2 to 20,000 mPa⋅s. The substance is stable at pH 2–12. |
| Hydroxypropyl cellulose (HPC) | Formed by the connection of propylene oxide to -OH groups in cellulose. | White- to slightly yellow-colored, odorless, inert, and tasteless powder. The viscosity of 2% of water solutions is from 2 to 6500 mPa⋅s. It is well soluble in water at temperatures below 38 °C, precipitating at temperatures > 40 °C. Solutions are stable at pH 6–8. HPC dissolves freely in cold water and provides a smooth, transparent, colloidal solution. It is insoluble in hot water and forms a flocs. HPC is used as a film-forming material due to its good pharmaceutical and mechanical properties. HPC films exhibit good loading capacity, transparency, and balanced bioadhesive performance associated with the swelling ability. |
| Hydroxypropylmethyl cellulose (HPMC) | Obtained by alkylation of cellulose with methyl chloride or a combination of methyl chloride and propylene oxide. | Soluble in water (below 60 °C) and in organic solvents. White, creamy, odorless, and tasteless powder. MW ranges from 10,000 to 1,500,000. The viscosity of 2% aqueous solution is from 2 to 200,000 mPa⋅s. Stable at pH 3–11. The use of HPMC is beneficial in increasing the solubility and dissolution of drugs with poor water solubility. |
| Substitution Type | Methoxy (%) | Hydroxypropoxy (%) | ||
|---|---|---|---|---|
| Min. | Max. | Min. | Max. | |
| 1828 | 16.5 | 20.0 | 23.0 | 32.0 |
| 2208 | 19.0 | 24.0 | 4.0 | 12.0 |
| 2906 | 27.0 | 30.0 | 4.0 | 7.5 |
| 2910 | 28.0 | 30.0 | 7.0 | 12.0 |
| Polymers | Other Excipients | Drug | Manufacturing Method | Comments/Observations |
|---|---|---|---|---|
| HPMC E3, E5, E15 | xantan gum, xylitol, glycerin, propylene glycol | triclosan | solvent casting | Placebo films were created from HPMC E3, E5, E15, but the most desired properties were shown for type E5 (at concentration 2.2%, w/v). Of the different film modifiers used (xanthan and guar gums, carrageen), xanthan gum exhibited the finest capacity to enhance the film-forming properties of HPMC. The addition of mannitol to the formulation resulted in opaque films, while films containing xylitol and sorbitol gave transparent product. Nevertheless, for film creation the preffered one was xylitol in regard to its more negative solution heat and lower hygroscopicity in comparison to sorbitol. It is expected that agents with a more negative solution heat would give a stronger cooling sensation in the mouth, which is important in taste-masking effect [43]. |
| pullulan, HPMC E5, HPMC E15 | PEG | granisetron hydrochloride | solvent casting | ODFs were developed from 2.5%, 5%, 7.5%, and 10% solutions of pullulan, HPMC E5, or HPMC E15. Pulluan and HPMC E15 showed the desired film-forming capacity while ODFs made of HPMC E5 possessed average film-forming properties and were semitransparent. An examination of the release of the drug and mechanical features revealed that for ODFs made of 5% pullulan granisetron release rate was the highest, about 92% of the amount of drug was released, and simultaneously the fastest [44]. |
| HPMC E5, HPC, PVA, SA | sucralose | anastrazole | solvent casting | Among the examined formulations, films obtained utilizing HPMC E5 showed shorter disintegration time (15 s) with satisfactory mechanical properties [45]. |
| HPMC E3 | glycerol 85%, carbomer 974 | influenza vaccine | solvent casting | Formulation of the proposed combination was advantageous as it provided improved stability for influenza vaccine and constituted a suitable, inert carrier for the drug [46]. |
| HPMC (Metolose 60SH-50)—10%, mesoporous colloidal silica—10%. | glycerol | haloperidol | solvent evaporation pour-off method | Haloperidol solution was printed on ODFs in the form of a QR code [47]. |
| HPMC | PEG 400, Na CMC | escitalopram | solvent casting | Different concentrations of HPMC were tested: 5%, 7.5%, 10%. The thickness of the formulations with polymer concentration 5% was assessed to be superior compared to formulations containing 7.5% and 10%. The viscosity of the solutions with higher polymer concentration was too high and the presence of lumps in the solution created a great challenge to spread [48]. |
| Pharmacoat 606 | glycerol 85%, PEG | loratadine hydrochloride | solvent casting | Disintegration time was mainly influenced by the polymer concentration (2.5%, 5%, or 10%): With the increased amount of polymer, increased disintegration time was observed. The shortest disintegration time was noted in formulations containing HPMC at concentration of 2.5%. ODFs containing glycerol as plasticizer possessed better mechanical strength compared to formulations with polyethylene glycol. The films containing polyethylene addition were characterized by greater moisture content and tended to stick together. Moreover, volunteers claimed that films with PEG or polyethylene glycol/glycerol blend were of unsavory taste. It was decided to use glycerol at a concentration of 2.5% to strike a balance between mechanical properties and taste-masking effect. Interestingly, a mixture of both plasticizers did not improve the mechanical properties of ODFs [17]. |
| HPMC E15 | pregelatinized starch, maltitol, sucralose | levocetirizine hydrochloride | semisolid extrusion 3D printer | The polymer was applied at concentration of 17% (choosen experimentally from 15%, 16%, 17%, 18%, 20% in terms of viscosity of solution that would be printed in a satisfactory manner). The resulting formulations showed optimal elasticity and rapid drug release in vitro by complete dissolution below 2 min [49]. |
| HPC:HPMC in 1:4 ratio; dextran maltodextrin | PEG 400, glycerol 85%, sorbitol, microcrystalline cellulose, CMC | amphotericin B | solvent casting | The optimised ODF composition consisted of 1% drug, 25% dextran, 25% maltodextrin, 5% sorbitol, 10% microcrystalline cellulose, 10% PEG 400, 10% glycerol, 3% HPM, and 12% HPMC. The formulation disintegrated quickly (60 s), providing quick release (>80% in 10 min) in artificial saliva [50]. |
| Polymer | Characteristic | Utilization in ODFs |
|---|---|---|
| Sodium alginate | Alginates are naturally occurring polysaccharides extensively utilized in the pharmaceutical industry because of their broad availability, safety, and biocompatibility They are sourced from various genera of brown algae (mainly Laminaria hyperborea, Macrocystis pyrifera, Ascophyllum nodosum and in lesser extent from Laminaria digitate, Laminaria japonica, Eclonia maxima, Lesonia nigrescens, Sargassum sp.) or synthesized by some bacteria such as Azotobacter vinelandii or mucoid strains of Pseudomonas aeruginosae. The bacterial alginates possess O-acetyl groups, whereas these are not contained in the structure of algal alginates. Moreover, bacterial alginates are characterized by higher molecular weights in comparison to the algal ones [18]. | Sodium alginate- and PVA-based ODFs with sildenafil citrate obtained by solvent-casting method were successfully developed and characterized by short disintegration time (20 s) [64]. Sodium alginate-, pectin-, and gelatin-based ODFs with zolmitriptan and propylene glycol as plasticizer were developed by solvent-casting method. According to the physical and mechanical properties, sodium alginate was selected as the best film former [65]. |
| Chitosan | The polymer is obtained by the deacetylation of chitin from crustacean shells, which consist of β-(1-4)-2-acetamido-D-glucose and β-(1-4)-2-amino-D-glucose units. This biodegradable polysaccharide possesses satisfying film-forming properties. Chitosan-based films show good permeability, biocompatibility, and advantageous pharmacological profile (antibacterial and antifungal properties of chitosan per se) [18,61,63]. | ODFs with donepezil formulated from chitosan blended with polyester were characterized by fast disintegration time and optimal drug release [66]. Chitosan and pullulan electrospun orodispersible films containing acetylsalicylic acid were developed with the total polymer content kept at 10% (w/v). The weight ratios of chitosan to pullulan in variously mixed blend solutions were 0:100, 10:90, 20:80, 30:70, 40:60, 50:50, and 60:40, accordingly. Since electrospinning of pure chitosan is troublesome, owing to its intra-molecular interactions, polycationic nature in solution, and rigid structure, pullulan was utilized to facilitate the electrospinnability of the solutions by raising viscosity and decreasing conductivity and superficial tension [67]. |
| Product | Polymer(s) | Additional Excipients | References |
|---|---|---|---|
| Drug | |||
| Benadryl Allergy quick dissolve strip Diphenhydramine | carragen, pullulan | glycerin, propylene glycol, acesulfam K | [81] |
| Chloraseptic Sore Throat Relief Strips Benzocaine | corn starch | erithrytol, malic acid, menthol, macrogol, sucralose | [82] |
| D-Fusion Film 800 UI Cholecalciferol | pullulan, HPC | xylitol, sucralose, glycerol | [83] |
| Exservan Oral film Riluzole | HPC | glycerol, glycerol monooleate, polacrilex resin, sucralose, xanthan gum, xylitol | [84] |
| Gas-X Thin Strips Simethicone | maltodextrin, HPMC | macrogol, sorbitol, sucralose | [85] |
| IvyFilm, IvyFilm Kiddies Hedera helix extract | Pullulan | glycerol, sucralose | [86] |
| Listerine PocketPaks Oral Care Strips Menthol | pullulan, carragenan, xanthan gum | menthol, aspartame, acesulfam K, macrogol | [87] |
| Niquitin Mint Oral Film Strips Nicotine | methacrylic acid—ethyl acrylate copolymer (1:1) | sucralose, sodium hydrogen carbonate, peppermint flavor | [80] |
| Pedia-Lax Quick Dissolve Strip Sennosides | HPMC | malic acid, glycerol, menthol sucralose | [88] |
| Sudafed PE Phenylephrine | maltodextrin, pullulan, carrageen | acesulfam K, aspartame, glycerin, macrogol | [89] |
| SildeHEXAL SF orodispersible film Sildenafil | HPMC | glycerol, sucralose, peppermint flavor, levomenthol | [90] |
| Sildenafil IBSA Orodispersible film Sildenafil | Maltodextrin | glycerol, polysorbat 20, propylene glycol, lemon and grapefruit flavors | [91] |
| Tusheel Hedera helix extract | pullulan, NaCM | glycerol, sucralose, | [92] |
| Setofilm Ondansetron | PVA | PVA, macrogol, levomenthol, glycerol | [93] |
| Sympazan oral film Clobazam | HPC | citric acid, glycerol monooleate, maltiotol | [94] |
| Zentrip Meclizine hydrochloride | HPMC | acesulfame potassium, mannitol, menthol, orange oil, polyethylene glycol 400, sucralose | [95] |
| Zuplenz Ondansetron | HPMC | erythrytol, sucralose, peppermint | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olechno, K.; Basa, A.; Winnicka, K. “Success Depends on Your Backbone”—About the Use of Polymers as Essential Materials Forming Orodispersible Films. Materials 2021, 14, 4872. https://doi.org/10.3390/ma14174872
Olechno K, Basa A, Winnicka K. “Success Depends on Your Backbone”—About the Use of Polymers as Essential Materials Forming Orodispersible Films. Materials. 2021; 14(17):4872. https://doi.org/10.3390/ma14174872
Chicago/Turabian StyleOlechno, Katarzyna, Anna Basa, and Katarzyna Winnicka. 2021. "“Success Depends on Your Backbone”—About the Use of Polymers as Essential Materials Forming Orodispersible Films" Materials 14, no. 17: 4872. https://doi.org/10.3390/ma14174872