Study on the Hemostasis Characteristics of Biomaterial Frustules Obtained from Diatom Navicula australoshetlandica sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diatom Species and Culture Conditions
2.2. Cultivation of Navicula australoshetlandica sp. in Artificial Aquaculture Wastewater
2.3. Preparation of Diatom Frustules
2.4. Physical and Chemical Characterization for Diatom Frustules
2.5. In Vitro Hemolysis Test
2.6. In Vitro Blood Clotting Evaluation
2.6.1. The Whole Blood Clotting Test
2.6.2. Blood Coagulation Tests
2.7. Analytical Methods
3. Results
3.1. Physical and Chemical Characteristics Analysis of Diatom Frustules
3.2. In Vitro Hemolysis Test
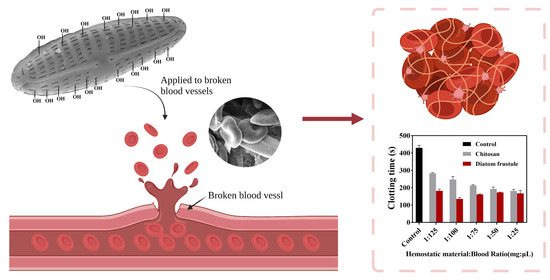
3.3. In Vitro Blood Clotting Evaluation
4. Discussion
4.1. In Vitro Clotting Times of Different Diatom Frustules
4.2. Surface Modification of Diatom Frustules Benefits Hemostatic Performance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakov, T.; Beaulieu, J.M.; Alverson, A.J. Diatoms diversify and turn over faster in freshwater than marine environments. Evolution 2019, 73, 2497–2511. [Google Scholar] [CrossRef]
- Thomas Kiran, M.; Itzel, Y.L.-P.; Roberto, P.-S.; Sreenath, D.; Archana, T. Wealth from waste: Diatoms as tools for phycoremediation of wastewater and for obtaining value from the biomass. Sci. Total Environ. 2020, 724, 137960. [Google Scholar] [CrossRef]
- Görlich, S.; Pawolski, D.; Zlotnikov, I.; Kröger, N. Control of biosilica morphology and mechanical performance by the conserved diatom gene Silicanin-1. Commun. Biol. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Managò, S.; Migliaccio, N.; Terracciano, M.; Napolitano, M.; Martucci, N.M.; De Stefano, L.; Rendina, I.; De Luca, A.C.; Lamberti, A.; Rea, I. Internalization kinetics and cytoplasmic localization of functionalized diatomite nanoparticles in cancer cells by Raman imaging. J. Biophotonics 2018, 11, e201700207. [Google Scholar] [CrossRef]
- Morales, L.V.; Sigman, D.M.; Horn, M.G.; Robinson, R.S. Cleaning methods for the isotopic determination of diatombound nitrogen in non-fossil diatom frustules. Limnol. Oceanogr. Methods 2013, 11, 101–112. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Cai, J.; Pan, J.; Chen, M.; Li, A.; Jiang, Y. Biosilica structures obtained from Nitzschia, Ditylum, Skeletonema, and Coscinodiscus diatom by a filtration-aided acid cleaning method. Appl. Microbiol. Biotechnol. 2012, 95, 1165–1178. [Google Scholar] [CrossRef]
- Uthappa, U.T.; Brahmkhatri, V.; Sriram, G.; Jung, H.-Y.; Yu, J.; Kurkuri, N.; Aminabhavi, T.M.; Altalhi, T.; Neelgund, G.M.; Kurkuri, M.D. Nature engineered diatom biosilica as drug delivery systems. J. Control. Release 2018, 281, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Delalat, B.; Sheppard, V.C.; Ghaemi, S.R.; Rao, S.; Prestidge, C.A.; McPhee, G.; Rogers, M.-L.; Donoghue, J.F.; Pillay, V.; Johns, T.G. Targeted drug delivery using genetically engineered diatom biosilica. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terracciano, M.; De Stefano, L.; Rea, I. Diatoms green nanotechnology for biosilica-based drug delivery systems. Pharmaceutics 2018, 10, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Stefano, L.; Lamberti, A.; Rotiroti, L.; De Stefano, M. Interfacing the nanostructured biosilica microshells of the marine diatom Coscinodiscus wailesii with biological matter. Acta Biomater. 2008, 4, 126–130. [Google Scholar] [CrossRef]
- Lulu, W.; Kehou, P.; Lin, Z.; Chengxu, Z.; Yun, L.; Baohua, Z.; Jichang, H. Tentative identification of key factors determining the hemostatic efficiency of diatom frustule. Biomater. Sci. 2021. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Haynes, C.L. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J. Am. Chem. Soc. 2010, 132, 4834–4842. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Ozgur, E.; Bayindir, M. Impact of mesoporous silica nanoparticle surface functionality on hemolytic activity, thrombogenicity and non-specific protein adsorption. J. Mater. Chem. B 2013, 1, 1909–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slowing, I.I.; Vivero-Escoto, J.L.; Trewyn, B.G.; Lin, V.S.-Y. Mesoporous silica nanoparticles: Structural design and applications. J. Mater. Chem. 2010, 20, 7924–7937. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.; Wang, J.; Boronat, M.; Yi, L.; Su, J.H.; Ju, H.; Ma, Y.; Yu, J. Radical-facilitated green synthesis of highly ordered mesoporous silica materials. J. Am. Chem. Soc. 2018, 140, 4770–4773. [Google Scholar] [CrossRef] [PubMed]
- Jeehee, L.; Haesung, A.L.; Mikyung, S.; Lih Jiin, J.; Christian, J.K.; Gyung Min, G.; Haeshin, L. Diatom frustule silica exhibits superhydrophilicity and superhemophilicity. ACS Nano 2020. [Google Scholar] [CrossRef]
- Chao, F.; Jing, L.; Guang Sheng, W.; Yu Zhi, M.; Ming, K.; Chang Qing, J.; Xiao Jie, C.; Ya, L.; Xi Guang, C. Chitosan-coated diatom silica as hemostatic agent for hemorrhage control. ACS Appl. Mater. Interfaces 2016, 8, 34234–34243. [Google Scholar] [CrossRef]
- Wang, L.; Pan, K.; Li, J.; Li, Y.; Zhu, B.; Wang, Y.; Feng, C.; Han, J. Influence of the physicochemical characteristics of diatom frustules on hemorrhage control. Biomater. Sci. 2019. [Google Scholar] [CrossRef]
- Stein, J.R. Handbook of Phycological Methods: Culture Methods and Growth Measurements; Cambridge University Press: Cambridge, UK, 1973. [Google Scholar]
- Mofrad, A.M.; Peixoto, C.; Blumeyer, J.; Liu, J.; Hunt, H.K.; Hammond, K.D. Vibrational spectroscopy of sodalite: Theory and experiments. J. Phys. Chem. C 2018, 122, 24765–24779. [Google Scholar] [CrossRef]
- Vishwas, M.; Narasimha Rao, K.; Phani, A.R.; Arjuna Gowda, K.V.; Chakradhar, R.P.S. Spectroscopic and electrical properties of SiO2 films prepared by simple and cost effective sol–gel process. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 695–699. [Google Scholar] [CrossRef]
- Yanbo, L.; Ran, W.; Wentan, R.; Yong, Z. The surface modification by carboxyl ionic liquid for mesoporous silica and the preparation of composite polymer electrolyte with the modified mesoporous silica. Polym. Compos. 2017. [Google Scholar] [CrossRef]
- Cristina, P.; Maura, T.; Mara, G.; Virginie, R.; Vera, B.; Dominique, L.; Bice, F. In search of the chemical basis of the hemolytic potential of silicas. Chem. Res. Toxicol. 2013, 26, 1188–1198. [Google Scholar] [CrossRef]
- Murashov, V.; Harper, M.; Demchuk, E. Impact of silanol surface density on the toxicity of silica aerosols measured by erythrocyte haemolysis. J. Occup. Environ. Hyg. 2006, 3, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, Y. Basic evaluation of typical nanoporous silica nanoparticles in being drug carrier: Structure, wettability and hemolysis. Mater. Sci. Eng. C 2017, 73, 670–673. [Google Scholar] [CrossRef]
- Shida, N.; Kurasawa, R.; Maki, Y.; Toyama, Y.; Dobashi, T.; Yamamoto, T. Study of plasma coagulation induced by contact with calcium chloride solution. Soft Matter 2016, 12, 9471–9476. [Google Scholar] [CrossRef]
- Okamoto, Y.; Yano, R.; Miyatake, K.; Tomohiro, I.; Shigemasa, Y.; Minami, S. Effects of chitin and chitosan on blood coagulation. Carbohydr. Polym. 2003, 53, 337–342. [Google Scholar] [CrossRef]
- Naito, K.; Fujikawa, K. Activation of human blood coagulation factor XI independent of factor XII. Factor XI is activated by thrombin and factor XIa in the presence of negatively charged surfaces. J. Biol. Chem. 1991. [Google Scholar] [CrossRef]
- Sperling, C.; Fischer, M.; Maitz, M.F.; Werner, C. Blood coagulation on biomaterials requires the combination of distinct activation processes. Biomaterials 2009, 30, 4447–4456. [Google Scholar] [CrossRef]
- Cicco, S.R.; Vona, D.; Gristina, R.; Sardella, E.; Ragni, R.; Lo Presti, M.; Farinola, G.M. Biosilica from living diatoms: Investigations on biocompatibility of bare and chemically modified Thalassiosira weissflogii silica shells. Bioengineering 2016, 3, 35. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.; Yuan, Y.; Liu, C.; Wei, J.; Hong, H.; Li, X.; Pan, X. Degradable, antibacterial silver exchanged mesoporous silica spheres for hemorrhage control. Biomaterials 2009, 30, 5364–5375. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Kadri, N.A.; Zeimaran, E.; Towler, M.R. Well-ordered mesoporous silica and bioactive glasses: Promise for improved hemostasis. Biomater. Sci. 2018, 7, 31–50. [Google Scholar] [CrossRef]
- Renné, T.; Schmaier, A.H.; Nickel, K.F.; Blombäck, M.; Maas, C. In vivo roles of factor XII. Blood J. Am. Soc. Hematol. 2012, 120, 4296–4303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, L.; Jichang, H.; Qingjie, S.; Yanan, W.; Yuzhi, M.; Kaichao, Z.; Xiaoyu, D.; Ming, K.; Xiguang, C.; Chao, F. Biosynthetic calcium-doped biosilica with multiple hemostatic properties for hemorrhage control†. J. Mater. Chem. B 2018, 6, 7834–7841. [Google Scholar] [CrossRef]
- Chen, Z.; Li, F.; Liu, C.; Guan, J.; Hu, X.; Du, G.; Yao, X.; Wu, J.; Tian, F. Blood clot initiation by mesoporous silica nanoparticles: Dependence on pore size or particle size? J. Mater. Chem. B 2016. [Google Scholar] [CrossRef]
- Jiang, W.; Luo, S.; Liu, P.; Deng, X.; Jing, Y.; Bai, C.; Li, J. Purification of biosilica from living diatoms by a two-step acid cleaning and baking method. J. Appl. Phycol. 2014, 26, 1511–1518. [Google Scholar] [CrossRef]
- Saad, E.M.; Pickering, R.A.; Shoji, K.; Hossain, M.I.; Glover, T.G.; Krause, J.W.; Tang, Y. Effect of cleaning methods on the dissolution of diatom frustules. Mar. Chem. 2020, 224, 103826. [Google Scholar] [CrossRef]
- Lim, G.W.; Lim, J.K.; Ahmad, A.L.; Chan, D.J.C. Influences of diatom frustule morphologies on protein adsorption behavior. J. Appl. Phycol. 2015, 27, 763–775. [Google Scholar] [CrossRef]
- Jing, L.; Xiaojie, S.; Kaichao, Z.; Guoning, Y.; Yuzhi, M.; Chang, S.; Jianhui, P.; Tongtong, C.; Xiguang, C.; Chao, F. Chitosan/Diatom-biosilica aerogel with controlled porous structure for rapid hemostasis. Adv. Healthc. Mater. 2020, 9, e2000951. [Google Scholar] [CrossRef]
- Yanan, W.; Yangmu, F.; Jing, L.; Yuzhi, M.; Xin, Z.; Kaichao, Z.; Mengqi, L.; Chao, F.; Xiguang, C. Multifunctional Chitosan/Dopamine/Diatom-Biosilica composite beads for rapid blood coagulation. Carbohydr. Polym. 2018, 200, 6–14. [Google Scholar] [CrossRef]
- Kaichao, Z.; Jing, L.; Yanan, W.; Yuzhi, M.; Xiaojie, S.; Chang, S.; Yao, D.; Jianhui, P.; Liang, H.; Xiguang, C.; et al. Hydroxybutyl Chitosan/Diatom-biosilica composite sponge for hemorrhage control. Carbohydr. Polym. 2020, 236, 116051. [Google Scholar] [CrossRef]
- Mu, Y.; Fu, Y.; Li, J.; Shao, K.; Pang, J.; Su, C.; Cai, Y.; Sun, X.; Cong, X.; Chen, X.; et al. Thrombin immobilized polydopamine-diatom biosilica for effective hemorrhage control. Biomater. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Aw, M.S.; Simovic, S.; Yu, Y.; Addai-Mensah, J.; Losic, D. Porous silica microshells from diatoms as biocarrier for drug delivery applications. Powder Technol. 2012, 223, 52–58. [Google Scholar] [CrossRef]
- Bariana, M.; Aw, M.S.; Kurkuri, M.; Losic, D. Tuning drug loading and release properties of diatom silica microparticles by surface modifications. Int. J. Pharm. 2013, 443, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Ekta, K.; Stefan, G.; Nicole, P.; Nils, K. Genetically programmed regioselective immobilization of enzymes in biosilica microparticles. Adv. Funct. Mater. 2020. [Google Scholar] [CrossRef]




| Analysis Adsorptive | Analysis Bath Temperature | Sample Mass | Warm Free Space | Cold Free Space | Equilibration Interval |
|---|---|---|---|---|---|
| N2 | 77.30 K | 0.0574 g | 17.38 cm³ | 49.99 cm³ | 30 s |
| SBET (m² g−1) | V (cm3 g−1) | WBJH (nm) | Wd (nm) | SBJH (m2 g−1) |
|---|---|---|---|---|
| 401.45 | 0.46 | 8.58 | 4.61 | 329.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Li, S.; Shen, K.; Song, Y.; Zhang, J.; Su, W.; Yang, X. Study on the Hemostasis Characteristics of Biomaterial Frustules Obtained from Diatom Navicula australoshetlandica sp. Materials 2021, 14, 3752. https://doi.org/10.3390/ma14133752
Luo Y, Li S, Shen K, Song Y, Zhang J, Su W, Yang X. Study on the Hemostasis Characteristics of Biomaterial Frustules Obtained from Diatom Navicula australoshetlandica sp. Materials. 2021; 14(13):3752. https://doi.org/10.3390/ma14133752
Chicago/Turabian StyleLuo, Yanqing, Shuangfei Li, Kun Shen, Yingjie Song, Jiangtao Zhang, Wen Su, and Xuewei Yang. 2021. "Study on the Hemostasis Characteristics of Biomaterial Frustules Obtained from Diatom Navicula australoshetlandica sp." Materials 14, no. 13: 3752. https://doi.org/10.3390/ma14133752