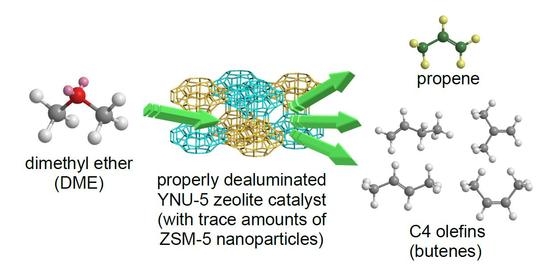
The Synthesis of YNU-5 Zeolite and Its Application to the Catalysis in the Dimethyl Ether-to-Olefin Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurements
2.2. Typical YNU-5 Synthesis Procedure
2.3. Preparation of MFI-Type Zeolite Nanocrystals
2.4. Post-Synthesis Modification
2.5. Procedure for the DTO Reaction
3. Results and Discussion
3.1. Synthetic Investigations of YNU-5
3.2. Effects of a Trace of MFI on the Physicochemical Properties of YFI Samples
3.3. DTO Reaction over the YFI Catalyst
3.3.1. Effect of MFI as a Trace Contaminant
3.3.2. Catalytic Characteristics of Pure YNU-5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Davis, M.E. Zeolites from a materials chemistry perspective. Chem. Mater. 2014, 26, 239–245. [Google Scholar] [CrossRef]
- Groen, J.C.; Bach, T.; Ziese, U.; Paulaime-van Donk, A.M.; de Jong, K.P.; Moulijn, J.A.; Pérez-Ramírez, J. Creation of hollow zeolite architectures by controlled desilication of Al-zoned ZSM-5 crystals. J. Am. Chem. Soc. 2005, 127, 10792–10793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Speybroeck, V.; Hemelsoet, K.; Joos, L.; Waroquier, M.; Bell, R.G.; Catlow, C.R. Advances in theory and their application within the field of zeolite chemistry. Chem. Soc. Rev. 2015, 44, 7044–7111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degnan, T.F., Jr. The implications of the fundamentals of shape selectivity for the development of catalysts for the petroleum and petrochemical industries. J. Catal. 2003, 216, 32–46. [Google Scholar] [CrossRef]
- Ruthven, D.M.; Reyes, S.C. Adsorptive separation of light olefins from paraffins. Microporous Mesoporous Mater. 2007, 104, 59–66. [Google Scholar] [CrossRef]
- Degnan, T.F., Jr. Applications of zeolites in petroleum refining. Top. Catal. 2000, 13, 349–356. [Google Scholar] [CrossRef]
- Camblor, M.A.; Barrett, P.A.; Díaz-Cabañas, M.; Villaescusa, L.A.; Puche, M.; Boix, T.; Pérez, E.; Koller, H. High silica zeolites with three-dimensional systems of large pore channels. Microporous Mesoporous Mater. 2001, 48, 11–22. [Google Scholar] [CrossRef]
- Reddy, K.S.N.; Rao, B.S.; Shiralkar, V.P. Alkylation of benzene with isopropanol over zeolite beta. Appl. Catal. A Gen. 1993, 95, 53–63. [Google Scholar] [CrossRef]
- Shamshoum, E.S.; Schuler, T.R.; Ghosh, A.K. Aromatic Alkylation Process Employing Steam Modified Zeolite Beta Catalyst. U.S. Patent 5,227,558, 13 July 1993. [Google Scholar]
- Gajda, G.J.; Gajek, R.T. Zeolite Beta and Its Use in Aromatic Alkylation. U.S. Patent 5723710A, 3 March 1998. [Google Scholar]
- Wadlinger, R.L.; Kerr, G.T.; Rosinski, E.J. Catalytic Composition of a Crystalline Zeolite. U.S. Patent 3,308,069, 7 March 1967. [Google Scholar]
- Corma, A.; Camblor, M.; Esteve, P.; Martinez, A.; Perezpariente, J. Activity of Ti-Beta catalyst for the selective oxidation of alkenes and alkanes. J. Catal. 1994, 145, 151–158. [Google Scholar] [CrossRef]
- Corma, A.; Nemeth, L.T.; Renz, M.; Valencia, S. Sn-zeolite beta as a heterogeneous chemoselective catalyst for Baeyer–Villiger oxidations. Nature 2001, 412, 423–425. [Google Scholar] [CrossRef]
- Lobo, R.F.; Davis, M.E. CIT-1: A new molecular sieve with intersecting pores bounded by 10-and 12-rings. J. Am. Chem. Soc. 1995, 117, 3766–3779. [Google Scholar] [CrossRef]
- Yoshioka, M.; Yokoi, T.; Tatsumi, T. Development of the CON-type aluminosilicate zeolite and its catalytic application for the MTO reaction. ACS Catal. 2015, 5, 4268–4275. [Google Scholar] [CrossRef]
- Elangovan, S.P.; Ogura, M.; Davis, M.E.; Okubo, T. SSZ-33: A promising material for use as a hydrocarbon trap. J. Phys. Chem. B 2004, 108, 13059–13061. [Google Scholar] [CrossRef]
- Mathew, T.; Elangovan, S.P.; Yokoi, T.; Tatsumi, T.; Ogura, M.; Kubota, Y.; Shimojima, A.; Okubo, T. Synthesis and characterization of aluminium containing CIT-1 and their structure–property relationship to hydrocarbon trap performance. Microporous Mesoporous Mater. 2010, 129, 126–135. [Google Scholar] [CrossRef]
- Kubota, H.; Liu, C.; Toyao, T.; Maeno, Z.; Ogura, M.; Nakazawa, N.; Inagaki, S.; Kubota, Y.; Shimizu, K. Formation and Reactions of NH4NO3 during Transient and Steady-state NH3–SCR of NOx over H-AFX Zeolites: Spectroscopic and Theoretical Studies. ACS Catal. 2020, 2334–2344. [Google Scholar] [CrossRef]
- Nakazawa, N.; Ikeda, T.; Hiyoshi, N.; Yoshida, Y.; Han, Q.; Inagaki, S.; Kubota, Y. A microporous aluminosilicate with 12-, 12-, and 8-ring pores and isolated 8-ring channels. J. Am. Chem. Soc. 2017, 139, 7989–7997. [Google Scholar] [CrossRef]
- Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 16 March 2020).
- Nakazawa, N.; Yoshida, Y.; Inagaki, S.; Kubota, Y. Synthesis of novel aluminosilicate YNU-5 and enhancement of the framework thermal stability by post-synthesis treatment. Microporous Mesoporous Mater. 2019, 280, 66–74. [Google Scholar] [CrossRef]
- Kawase, R.; Iida, A.; Kubota, Y.; Komura, K.; Sugi, Y.; Oyama, K.; Itoh, H. Hydrothermal synthesis of calcium and boron containing MFI-type zeolites by using organic amine as structure directing agent. Ind. Eng. Chem. Res. 2007, 46, 1091–1098. [Google Scholar] [CrossRef]
- Kubota, Y.; Tawada, S.; Nakagawa, K.; Naitoh, C.; Sugimoto, N.; Fukushima, Y.; Hanaoka, T.; Imada, Y.; Sugi, Y. Synthetic investigation of CIT-5 catalyst. Microporous Mesoporous Mater. 2000, 37, 291–301. [Google Scholar] [CrossRef]
- Inagaki, S.; Shinoda, S.; Kaneko, Y.; Takechi, K.; Komatsu, R.; Tsuboi, Y.; Yamazaki, H.; Kondo, J.; Kubota, Y. Facile fabrication of ZSM-5 zeolite catalyst with high durability to coke formation during catalytic cracking of paraffins. ACS Catal. 2013, 3, 74–78. [Google Scholar] [CrossRef]
- Inagaki, S.; Tsuboi, Y.; Nishita, Y.; Syahylah, T.; Wakihara, T.; Kubota, Y. Rapid Synthesis of an Aluminum-Rich MSE-Type Zeolite by the Hydrothermal Conversion of an FAU-Type Zeolite. Chem. Eur. J. 2013, 19, 7780–7786. [Google Scholar] [CrossRef]
- Maekawa, H.; Kubota, Y.; Sugi, Y. Hydrothermal synthesis of [Al]-SSZ-24 from [Al]-beta zeolite ([Al]-BEA) as precursors. Chem. Lett. 2004, 33, 1126–1127. [Google Scholar] [CrossRef]
- Kubota, Y.; Maekawa, H.; Miyata, S.; Tatsumi, T.; Sugi, Y. Hydrothermal synthesis of metallosilicate SSZ-24 from metallosilicate beta as precursors. Microporous Mesoporous Mater. 2007, 101, 115–126. [Google Scholar] [CrossRef]
- Ahedi, R.K.; Kubota, Y.; Sugi, Y. Synthesis of [Al]-SSZ-31 molecular sieves using [Al]-beta zeolite ([Al]-BEA) as precursors. Bull. Chem. Soc. Jpn. 2003, 76, 883–890. [Google Scholar] [CrossRef]
- Niwa, M.; Katada, N. Measurements of acidic property of zeolites by temperature programmed desorption of ammonia. Catal. Surv. Jpn. 1997, 1, 215–226. [Google Scholar] [CrossRef]
- Niwa, M.; Katada, N. New Method for the Temperature-Programmed Desorption (TPD) of Ammonia Experiment for Characterization of Zeolite Acidity: A Review. Chem. Rec. 2013, 13, 432–455. [Google Scholar] [CrossRef]
- Park, S.; Watanabe, Y.; Nishita, Y.; Fukuoka, T.; Inagaki, S.; Kubota, Y. Catalytic conversion of dimethyl ether into propylene over MCM-68 zeolite. J. Catal. 2014, 319, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Sawa, M.; Niwa, M.; Murakami, Y. Relationship between acid amount and framework aluminum content in mordenite. Zeolite 1990, 10, 532–538. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Ateka, A.; Pérez-Uriarte, P.; Castaño, P.; Aguayo, A.T.; Bilbao, J. Insight into the deactivation and regeneration of HZSM-5 zeolite catalysts in the conversion of dimethyl ether to olefins. Ind. Eng. Chem. Res. 2018, 57, 13689–13702. [Google Scholar] [CrossRef]
- Seidel, C.; Jörke, A.; Vollbrecht, B.; Seidel-Morgenstern, A.; Kienle, A. Kinetic modeling of methanol synthesis from renewable resources. Chem. Eng. Sci. 2018, 175, 130–138. [Google Scholar] [CrossRef]
- Al-Dughaither, A.S.; de Lasa, H. Neat dimethyl ether conversion to olefins (DTO) over HZSM-5: Effect of SiO2/Al2O3 on porosity, surface chemistry, and reactivity. Fuel 2014, 138, 52–64. [Google Scholar] [CrossRef]
- Deimund, M.A.; Harrison, L.; Lunn, J.D.; Liu, Y.; Malek, A.; Shayib, R.; Davis, M.E. Effect of heteroatom concentration in SSZ-13 on the methanol-to-olefins reaction. ACS Catal. 2016, 6, 542–550. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, S.; Song, Y.; Xu, L. Catalytic cracking of C4 alkenes to propene and ethene: Influences of zeolites pore structures and Si/Al2 ratios. Appl. Catal. A Gen. 2005, 288, 134–142. [Google Scholar] [CrossRef]
- Blay, V.; Epelde, E.; Miravalles, R.; Perea, L.A. Converting olefins to propene: Ethene to propene and olefin cracking. Catal. Rev. 2018, 60, 278–335. [Google Scholar] [CrossRef]
- Boulens, P.; Pellier, E.; Jeanneau, E.; Reek, J.N.; Olivier-Bourbigou, H.; Breuil, P.A.R. Self-assembled organometallic nickel complexes as catalysts for selective dimerization of ethylene into 1-butene. Organometallics 2015, 34, 1139–1142. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Yoshida, Y.; Nakazawa, N.; Inagaki, S.; Kubota, Y. The Synthesis of YNU-5 Zeolite and Its Application to the Catalysis in the Dimethyl Ether-to-Olefin Reaction. Materials 2020, 13, 2030. https://doi.org/10.3390/ma13092030
Liu Q, Yoshida Y, Nakazawa N, Inagaki S, Kubota Y. The Synthesis of YNU-5 Zeolite and Its Application to the Catalysis in the Dimethyl Ether-to-Olefin Reaction. Materials. 2020; 13(9):2030. https://doi.org/10.3390/ma13092030
Chicago/Turabian StyleLiu, Qing, Yuka Yoshida, Naoto Nakazawa, Satoshi Inagaki, and Yoshihiro Kubota. 2020. "The Synthesis of YNU-5 Zeolite and Its Application to the Catalysis in the Dimethyl Ether-to-Olefin Reaction" Materials 13, no. 9: 2030. https://doi.org/10.3390/ma13092030