Functional Characteristics and Mechanical Performance of PCU Composites for Knee Meniscus Replacement
Abstract
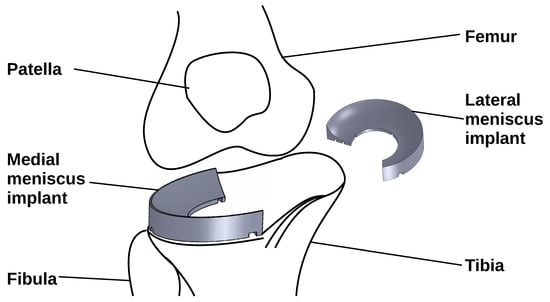
:1. Introduction
2. Materials and Methods
2.1. Materials and Processing
2.2. Composite Preparation
2.3. Mechanical Evaluation
2.4. Microstructural Analysis
3. Results and Discussion
3.1. Microstructural Characterization
3.2. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Majd, S.E.; Rizqy, A.I.; Kaper, H.J.; Schmidt, T.A.; Kuijer, R.; Sharma, P.K. An in vitro study of cartilage–meniscus tribology to understand the changes caused by a meniscus implant. Colloids Surf. B Biointerfaces 2017, 155, 294–303. [Google Scholar] [CrossRef]
- McDermott, I.D.; Masouros, S.D.; Amis, A.A. Biomechanics of the menisci of the knee. Curr. Orthop. 2008, 22, 193–201. [Google Scholar] [CrossRef]
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The knee meniscus: Structure–function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voloshin, A.S.; Wosk, J. Shock absorption of meniscectomized and painful knees: A comparative in vivo study. J. Biomed. Eng. 1983, 5, 157–161. [Google Scholar] [CrossRef]
- Vrancken, A.C.T.; Buma, P.; van Tienen, T.G. Synthetic meniscus replacement: A review. Int. Orthop. 2013, 37, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrett, W.E.J.; Swiontkowski, M.F.; Weinstein, J.N.; Callaghan, J.; Rosier, R.N.; Berry, D.J.; Harrast, J.; Derosa, G.P. American Board of Orthopedic Surgery Practice of the Orthopedic Surgeon: Part-II, Certification Examination Case Mix. J. Bone Jt. Surg. Am. 2006, 88, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Susanne, H.; Klaus, S. Epidemiology of athletic knee injuries: A 10-year study. Knee 2006, 13, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Baynat, C.; Andro, C.; Vincent, J.P.; Schiele, P.; Buisson, P.; Dubrana, F.; Gunepin, F.X. Actifit® synthetic meniscal substitute: Experience with 18 patients in Brest, France. Orthop. Traumatol. Surg. Res. 2014, 100, S385–S389. [Google Scholar] [CrossRef] [Green Version]
- McDermott, I.D.; Amis, A.A. The consequences of meniscectomy. J. Bone Jt. Surg. Br. 2006, 88, 1549–1556. [Google Scholar] [CrossRef] [Green Version]
- Lohmander, L.S.; Englund, P.M.; Dahl, L.L.; Roos, E.M. The Long-term Consequence of Anterior Cruciate Ligament and Meniscus Injuries: Osteoarthritis. Am. J. Sports Med. 2007, 35, 1756–1769. [Google Scholar] [CrossRef] [Green Version]
- Messner, K.; Gillquist, J. Prosthetic replacement of the rabbit medial meniscus. J. Biomed. Mater. Res. 1993, 27, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- McCann, L.; Ingham, E.; Jin, Z.; Fisher, J. Influence of the meniscus on friction and degradation of cartilage in the natural knee joint. Osteoarthr. Cartil. 2009, 17, 995–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Adams, J.; Athanasiou, K.A. The Knee Meniscus: A Complex Tissue of Diverse Cells. Cell. Mol. Bioeng. 2009, 2, 332. [Google Scholar] [CrossRef]
- De Bruycker, M.; Verdonk, P.C.; Verdonk, R.C. Meniscal allograft transplantation: A meta-analysis. SICOT-J. 2017, 3, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, H.; Fatih Cengiz, I.; Gomes, S.; Espregueira-Mendes, J.; Ripoll, P.L.; Monllau, J.C.; Reis, R.L.; Oliveira, J.M. Meniscal allograft transplants and new scaffolding techniques. EFORT Open Rev. 2019, 4, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Sweigart, M.A.; Athanasiou, K.A. Toward Tissue Engineering of the Knee Meniscus. Tissue Eng. 2001, 7, 111–129. [Google Scholar] [CrossRef]
- Zaffagnini, S.; Giordano, G.; Vascellari, A.; Bruni, D.; Neri, M.P.; Iacono, F.; Kon, E.; Presti, M.L.; Marcacci, M. Arthroscopic collagen meniscus implant results at 6 to 8 years follow up. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 175–183. [Google Scholar] [CrossRef]
- Bulgheroni, P.; Murena, L.; Ratti, C.; Bulgheroni, E.; Ronga, M.; Cherubino, P. Follow-up of collagen meniscus implant patients: Clinical, radiological, and magnetic resonance imaging results at 5years. Knee 2010, 17, 224–229. [Google Scholar] [CrossRef]
- Mandal, B.B.; Park, S.-H.; Gil, E.S.; Kaplan, D.L. Multilayered silk scaffolds for meniscus tissue engineering. Biomaterials 2011, 32, 639–651. [Google Scholar] [CrossRef] [Green Version]
- Scotti, C.; Hirschmann, M.T.; Antinolfi, P.; Martin, I.; Peretti, G.M. Meniscus repair and regeneration: Review on current methods and research potential. Eur. Cells Mater. 2013, 26, 150–170. [Google Scholar] [CrossRef]
- Sgaglione, N.A.; Steadman, J.R.; Shaffer, B.; Miller, M.D.; Fu, F.H. Current Concepts in Meniscus Surgery: Resection to Replacement. Arthrosc. - J. Arthrosc. Relat. Surg. 2003, 19, 161–188. [Google Scholar] [CrossRef] [PubMed]
- Chiari, C.; Koller, U.; Dorotka, R.; Eder, C.; Plasenzotti, R.; Lang, S.; Ambrosio, L.; Tognana, E.; Kon, E.; Salter, D.; et al. A tissue engineering approach to meniscus regeneration in a sheep model. Osteoarthr. Cartil. 2006, 14, 1056–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kun, M.; Chan, C.; Ramakrishna, S.; Kulkarni, A.; Vadodaria, K. 12—Textile-based scaffolds for tissue engineering. In The Textile Institute Book Series; Woodhead Publishing: Sawston, Cambridge, UK, 2019; pp. 329–362. ISBN 978-0-08-102192-7. [Google Scholar]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues—State of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Messner, K. Meniscal substitution with a Teflon-periosteal composite graft: A rabbit experiment. Biomaterials 1994, 15, 223–230. [Google Scholar] [CrossRef]
- Sommerlath, K.; Gallino, M.; Gillquist, J. Biomechanical characteristics of different artificial substitutes for rabbit medial meniscus and effect of prosthesis size on knee cartilage. Clin. Biomech. 1992, 7, 97–103. [Google Scholar] [CrossRef]
- Kang, S.-W.; Sun-Mi, S.; Jae-Sun, L.; Eung-Seok, L.; Kwon-Yong, L.; Sang-Guk, P.; Jung-Ho, P.; Byung-Soo, K. Regeneration of whole meniscus using meniscal cells and polymer scaffolds in a rabbit total meniscectomy model. J. Biomed. Mater. Res. Part A 2006, 77A, 659–671. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Tschon, M.; Fini, M.; Giavaresi, G.; Reggiani, L.M.; Chiari, C.; Nehrer, S.; Martin, I.; Salter, D.M.; et al. Tissue Engineering for Total Meniscal Substitution: Animal Study in Sheep Model—Results at 12 Months. Tissue Eng. Part A 2012, 18, 1573–1582. [Google Scholar] [CrossRef]
- Balint, E.; Gatt, C.J., Jr.; Dunn, M.G. Design and mechanical evaluation of a novel fiber-reinforced scaffold for meniscus replacement. J. Biomed. Mater. Res. Part A 2012, 100A, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merriam, A.R.; Patel, J.M.; Culp, B.M.; Gatt, C.J.; Dunn, M.G. Successful Total Meniscus Reconstruction Using a Novel Fiber-Reinforced Scaffold: A 16- and 32-Week Study in an Ovine Model. Am. J. Sports Med. 2015, 43, 2528–2537. [Google Scholar] [CrossRef]
- Patel, J.M.; Merriam, A.R.; Culp, B.M.; Gatt, C.J.; Dunn, M.G. One-Year Outcomes of Total Meniscus Reconstruction Using a Novel Fiber-Reinforced Scaffold in an Ovine Model. Am. J. Sports Med. 2016, 44, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Toguchida, J.; Oka, M. Development of an artificial meniscus using polyvinyl alcohol-hydrogel for early return to, and continuance of, athletic life in sportspersons with severe meniscus injury. I: Mechanical evaluation. Knee 2003, 10, 47–51. [Google Scholar] [CrossRef]
- Kobayashi, M.; Chang, Y.-S.; Oka, M. A two year in vivo study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials 2005, 26, 3243–3248. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.T.; Robertson, W.; Potter, H.G.; Deng, X.-H.; Turner, A.S.; Lyman, S.; Warren, R.F.; Rodeo, S.A. Hydrogel Meniscal Replacement in the Sheep Knee: Preliminary Evaluation of Chondroprotective Effects. Am. J. Sports Med. 2007, 35, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Holloway, J.L.; Lowman, A.M.; Palmese, G.R. Mechanical evaluation of poly(vinyl alcohol)-based fibrous composites as biomaterials for meniscal tissue replacement. Acta Biomater. 2010, 6, 4716–4724. [Google Scholar] [CrossRef] [PubMed]
- Elsner, J.J.; Portnoy, S.; Zur, G.; Guilak, F.; Shterling, A.; Linder-Ganz, E. Design of a Free-Floating Polycarbonate-Urethane Meniscal Implant Using Finite Element Modeling and Experimental Validation. J. Biomech. Eng. 2010, 132, 095001. [Google Scholar] [CrossRef] [PubMed]
- Holloway, J.L. Development and Characterization of UHMWPE Fiber-Reinforced Hydrogels for Meniscal Replacement. Ph.D. Thesis, Drexel University, Philadelphia, PA, USA, 2012. [Google Scholar]
- Holloway, J.L.; Lowman, A.M.; VanLandingham, M.R.; Palmese, G.R. Interfacial optimization of fiber-reinforced hydrogel composites for soft fibrous tissue applications. Acta Biomater. 2014, 10, 3581–3589. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Yan, W.; Xu, W. Application of the Finite Element Method in Implant Dentistry; Advanced Topics in Science and Technology in China; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 9783540737643. [Google Scholar]
- Shriram, D.; Praveen Kumar, G.; Cui, F.; Lee, Y.H.D.; Subburaj, K. Evaluating the effects of material properties of artificial meniscal implant in the human knee joint using finite element analysis. Sci. Rep. 2017, 7, 6011. [Google Scholar] [CrossRef]
- Trebše, R.; Mihelič, A. Joint Replacement: Historical Overview BT—Infected Total Joint Arthroplasty: The Algorithmic Approach; Trebše, R., Ed.; Springer: London, UK, 2012; pp. 7–11. ISBN 978-1-4471-2482-5. [Google Scholar]
- Oonishi, H.; Kadoya, Y.; Masuda, S. Gamma-irradiated cross-linked polyethylene in total hip replacements—Analysis of retrieved sockets after long-term implantation. J. Biomed. Mater. Res. 2001, 58, 167–171. [Google Scholar] [CrossRef]
- Kutzner, I.; Heinlein, B.; Graichen, F.; Bender, A.; Rohlmann, A.; Halder, A.; Beier, A.; Bergmann, G. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J. Biomech. 2010, 43, 2164–2173. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Craig, C.L.; Brown, W.J.; Clemes, S.A.; De Cocker, K.; Giles-Corti, B.; Hatano, Y.; Inoue, S.; Matsudo, S.M.; Mutrie, N.; et al. How many steps/day are enough? for adults. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Pangborn, C.A.; Athanasiou, K.A. Knee Meniscus, Biomechanics of. In Wiley Encyclopedia of Biomedical Engineering, 1st ed.; Akay, M., Ed.; Wiley-Interscience: Hoboken, NJ, USA, 28 April 2006. [Google Scholar]
- Abbot, A.E.; Levine, W.N.; Mow, V.C. Biomechanics of articular cartilage and menisci of the adult knee. In The Adult Knee; Callaghan, J.J., Rosenberg, A.G., Rubash, H.E., Simonian, P.T., Wickiewicz, T.L., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003; pp. 81–104. [Google Scholar]
- Zhang, J. Surface modification of ultra-high-molecular-weight polyethylene by argon plasma. J. Thermoplast. Compos. Mater. 2014, 27, 758–764. [Google Scholar]
- Riveiro, A.; Soto, R.; Del Val, J.; Comesaña, R.; Boutinguiza, M.; Quintero, F.; Lusquiños, F.; Pou, J. Laser surface modification of ultra-high-molecular-weight polyethylene (UHMWPE) for biomedical applications. Appl. Surf. Sci. 2014, 302, 236–242. [Google Scholar] [CrossRef]
- Ge, S.; Wang, S.; Huang, X. Increasing the wear resistance of UHMWPE acetabular cups by adding natural biocompatible particles. Wear 2009, 267, 770–776. [Google Scholar] [CrossRef]
- Plumlee, K.; Schwartz, C.J. Improved wear resistance of orthopedic UHMWPE by reinforcement with zirconium particles. Wear 2009, 267, 710–717. [Google Scholar] [CrossRef]
- Wright, J.I. Using Polyurethanes in Medical Applications. Med. Device Diagn. Ind. 2006, 28, 98–109. [Google Scholar]
- Christenson, E.M.; Dadsetan, M.; Wiggins, M.; Anderson, J.M.; Hiltner, A. Poly(carbonate urethane) and poly(ether urethane) biodegradation: In vivo studies. J. Biomed. Mater. Res. Part A 2004, 69A, 407–416. [Google Scholar] [CrossRef]
- Khan, I.; Smith, N.; Jones, E.; Finch, D.S.; Cameron, R.E. Analysis and evaluation of a biomedical polycarbonate urethane tested in an in vitro study and an ovine arthroplasty model. Part I: Materials selection and evaluation. Biomaterials 2005, 26, 621–631. [Google Scholar] [CrossRef]
- Khan, I.; Smith, N.; Jones, E.; Finch, D.S.; Cameron, R.E. Analysis and evaluation of a biomedical polycarbonate urethane tested in an in vitro study and an ovine arthroplasty model. Part II: In vivo investigation. Biomaterials 2005, 26, 633–643. [Google Scholar] [CrossRef]
- Geary, C.; Birkinshaw, C.; Jones, E. Characterisation of Bionate polycarbonate polyurethanes for orthopedic applications. J. Mater. Sci. Mater. Med. 2008, 19, 3355–3363. [Google Scholar] [CrossRef]
- van Tienen, T.G.; Hannink, G.; Buma, P. Meniscus Replacement Using Synthetic Materials. Clin. Sports Med. 2009, 28, 143–156. [Google Scholar] [CrossRef]
- Scholes, S.C.; Burgess, I.C.; Marsden, H.R.; Unsworth, A.; Jones, E.; Smith, N. Compliant Layer Acetabular Cups: Friction Testing of a Range of Materials and Designs for a New Generation of Prosthesis that Mimics the Natural Joint. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2006, 220, 583–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.L.; Ash, H.E.; Unsworth, A. A tribological study of UHMWPE acetabular cups and polyurethane compliant layer acetabular cups. J. Biomed. Mater. Res. 2000, 53, 710–716. [Google Scholar] [CrossRef]
- Caravia, L.; Dowson, D.; Fisher, J. Start up and steady state friction of thin polyurethane layers. Wear 1993, 160, 191–197. [Google Scholar] [CrossRef]
- Inyang, A.O.; Abdalrahman, T.; Bezuidenhout, D.; Bowen, J.; Vaughan, C.L. Suitability of developed composite materials for meniscal replacement: Mechanical, friction and wear evaluation. J. Mech. Behav. Biomed. Mater. 2019, 89, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, R.W.; Manson, J.A. Fatigue of Engineering Plastics; Academic Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Ford, A.C.; Gramling, H.; Li, S.C.; Sov, J.V.; Srinivasan, A.; Pruitt, L.A. Micromechanisms of fatigue crack growth in polycarbonate polyurethane: Time dependent and hydration effects. J. Mech. Behav. Biomed. Mater. 2018, 79, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Jian, K.; Chen, Z.-H.; Ma, Q.-S.; Zheng, W.-W. Effects of pyrolysis processes on the microstructures and mechanical properties of Cf/SiC composites using polycarbosilane. Mater. Sci. Eng. A 2005, 390, 154–158. [Google Scholar] [CrossRef]
- Inyang, A.O.; Abdalrahman, T.; Vaughan, C.L. Novel composites for human meniscus replacement. In Orthop Biomaterials—Advances and Applications; Li, B., Webster, T., Eds.; Springer Pub Company, Inc.: Berlin/Heidelberg, Germany, 2018; pp. 547–568. [Google Scholar]
- Almarza, A.J.; Athanasiou, K.A. Design Characteristics for the Tissue Engineering of Cartilaginous Tissues. Ann. Biomed. Eng. 2004, 32, 2–17. [Google Scholar] [CrossRef]
- Tissakht, M.; Ahmed, A.M. Tensile stress-strain characteristics of the human meniscal material. J. Biomech. 1995, 28, 411–422. [Google Scholar] [CrossRef]
- Fithian, D.C.; Kelly, M.A.; Mow, V.C. Material properties and structure-function relationships in the menisci. Clin. Orthop. Relat. Res. 1990, 19–31. [Google Scholar] [CrossRef]
- Proctor, C.S.; Schmidt, M.B.; Whipple, R.R.; Kelly, M.A.; Mow, V.C. Material properties of the normal medial bovine meniscus. J. Orthop. Res. 1989, 7, 771–782. [Google Scholar] [CrossRef]
- Heijkants, R.G.J.C.; van Calck, R.V.; de Groot, J.H.; Pennings, A.J.; Schouten, A.J.; van Tienen, T.G.; Ramrattan, N.; Buma, P.; Veth, R.P.H. Design, synthesis and properties of a degradable polyurethane scaffold for meniscus regeneration. J. Mater. Sci. Mater. Med. 2004, 15, 423–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whipple, R.R.; Wirth, C.R.; Mow, V.C. Mechanical properties of the meniscus. In Advances in Bioengineering; Spilker, R.L., Ed.; ASME Press: New York, NY, USA, 1984; pp. 32–33. [Google Scholar]
- Rennie, W.J.; Finlay, D.B.L. Meniscal Extrusion in Young Athletes: Associated Knee Joint Abnormalities. Am. J. Roentgenol. 2006, 186, 791–794. [Google Scholar] [CrossRef]
- Sweigart, M.A.; Zhu, C.F.; Burt, D.M.; deHoll, P.D.; Agrawal, C.M.; Clanton, T.O.; Athanasiou, K.A. Intraspecies and Interspecies Comparison of the Compressive Properties of the Medial Meniscus. Ann. Biomed. Eng. 2004, 32, 1569–1579. [Google Scholar] [CrossRef]
- Chia, H.N.; Hull, M.L. Compressive moduli of the human medial meniscus in the axial and radial directions at equilibrium and at a physiological strain rate. J. Orthop. Res. 2008, 26, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.D.; Suh, J.-K.; Marui, T.; Woo, S.L.-Y. Interspecies variation of compressive biomechanical properties of the meniscus. J. Biomed. Mater. Res. 1995, 29, 823–828. [Google Scholar] [CrossRef]
- Bruni, D.; Iacono, F.; Akkawi, I.; Gagliardi, M.; Zaffagnini, S.; Marcacci, M. Unicompartmental knee replacement: A historical overview. Joints 2013, 1, 45–47. [Google Scholar] [PubMed]
- Harris, B. (Ed.) Fatigue in Composites: Science and Technology of the Fatigue Response of Fiber-Reinforced Plastics; Woodhead Publishing: Sawston, Cambridge, UK, 2003. [Google Scholar]
- Neppalli, R.; Marega, C.; Marigo, A.; Bajgai, M.P.; Kim, H.Y.; Causin, V. Poly(ε-caprolactone) filled with electrospun nylon fibers: A model for a facile composite fabrication. Eur. Polym. J. 2010, 46, 968–976. [Google Scholar] [CrossRef]
- Atuanya, C.U.; Edokpia, R.O.; Aigbodion, V.S. The physio-mechanical properties of recycled low density polyethylene (RLDPE)/bean pod ash particulate composites. Results Phys. 2014, 4, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Sanadi, A.R.; Caulfield, D.F.; Jacobson, R.E.; Rowell, R.M. Renewable Agricultural Fibers as Reinforcing Fillers in Plastics: Mechanical Properties of Kenaf Fiber-Polypropylene Composites. Ind. Eng. Chem. Res. 1995, 34, 1889–1896. [Google Scholar] [CrossRef]
- Shuhadah, S.; Supri, A.G. LDPE-Isophthalic Acid-Modified Egg Shell Powder Composites (LDPE/ESP I). J. Phys. Sci. 2009, 20, 87–98. [Google Scholar]









| Material Property | 80A | 90A | PE Fiber |
|---|---|---|---|
| Tear Strength (kN/m) | 64.90 | 96.40 | — |
| Ultimate Tensile Strength (MPa) | 46.64 | 55.11 | — |
| Density (kg/m3) | 1190 | 1200 | 960 |
| Elastic Modulus (MPa) | 12 | 29 | 126,000 |
| Melting temperature (°C) | — | — | 220 |
| Diameter (mm) | — | — | 0.31 |
| Elongation at break (%) | 531 | 406 | — |
| Matrix | Fiber | Specimen Type |
|---|---|---|
| Bionate 80A | — | MX1 |
| PE | MP1 | |
| Bionate 90A | — | MX2 |
| PE | MP2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inyang, A.O.; Vaughan, C.L. Functional Characteristics and Mechanical Performance of PCU Composites for Knee Meniscus Replacement. Materials 2020, 13, 1886. https://doi.org/10.3390/ma13081886
Inyang AO, Vaughan CL. Functional Characteristics and Mechanical Performance of PCU Composites for Knee Meniscus Replacement. Materials. 2020; 13(8):1886. https://doi.org/10.3390/ma13081886
Chicago/Turabian StyleInyang, Adijat Omowumi, and Christopher Leonard Vaughan. 2020. "Functional Characteristics and Mechanical Performance of PCU Composites for Knee Meniscus Replacement" Materials 13, no. 8: 1886. https://doi.org/10.3390/ma13081886