Improvement of Manganese Feroxyhyte’s Surface Charge with Exchangeable Ca Ions to Maximize Cd and Pb Uptake from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Adsorbents
2.2. Characterization
2.3. Adsorption Experiments
2.4. Leaching Behavior
3. Results and Discussion
3.1. Structural and Physicochemical Characterization
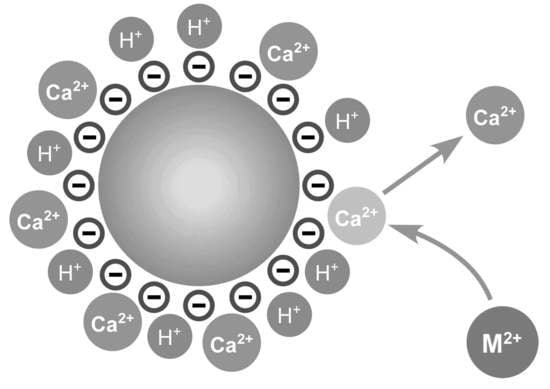
3.2. Modification of Surface Charge
3.3. Cadmium and Lead Speciation
3.4. Adsorption Isotherms
3.4.1. Cd Adsorption
3.4.2. Pb Adsorption
3.5. Column Tests
TMFx-Ca Consumption:
3.6. Leaching Tests
4. Conclusions
- ✓
- The negative surface charge density (mmol H+/g) of the applied adsorbent.
- ✓
- The ionic radius of metals (Mn+), which is related to the M–O distance of the relevant aqua complexes [M(H2O)yx+] and to the hydration free energy. A larger ionic radius (as in the case of Pb) results to greater M-O distance and to lower hydration free energy, which in turn can favor the improved selectivity and the higher QDWRL-value.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. Decision No. 2455/2001/EC of the European Parliament and of the Council establishing the list of priority substances in the field of water policy and amending Directive 2000/60/EC. Off. J. Eur. Union L 331. 2001. Available online: http://eur-lex.europa.eu/pri/en/oj/dat/2003/l_285/l_28520031101en00330037.pdf. (accessed on 12 December 2019).
- DIRECTIVE 98/8/EC concerning the placing of biocidal products on the market. Off. J. Eur. Communities 1998, 23, 3–65.
- Kokkinos, E.; Soukakos, K.; Kostoglou, M.; Mitrakas, M. Cadmium, mercury, and nickel adsorption by tetravalent manganese feroxyhyte: Selectivity, kinetic modeling, and thermodynamic study. Environ. Sci. Pollut. Res. 2018, 25, 12263–12273. [Google Scholar] [CrossRef] [PubMed]
- Simeonidis, K.; Mourdikoudis, S.; Kaprara, E.; Mitrakas, M.; Polavarapu, L. Inorganic engineered nanoparticles in drinking water treatment: A critical review. Environ. Sci. Water Res. Technol. 2016, 2, 43–70. [Google Scholar] [CrossRef] [Green Version]
- Bora, A.J.; Dutta, R.K. Removal of metals (Pb, Cd, Cu, Cr, Ni, and Co) from drinking water by oxidation-coagulation-absorption at optimized pH. J. Water Process Eng. 2019, 31, 100839–100847. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, H.; Zhang, G.; Li, J.; Yang, Y.; Ji, P. Potential of removing Cd(II) and Pb(II) from contaminated water using a newly modified fly ash. Chemosphere 2020, 242, 125148–125157. [Google Scholar] [CrossRef]
- Baruah, A.; Mondal, S.; Sahoo, L.; Gautam, U.K. Ni-Fe-layered double hydroxide/N-doped graphene oxide nanocomposite for the highly efficient removal of Pb(II) and Cd(II) ions from water. J. Solid State Chem. 2019, 280, 120963–120969. [Google Scholar] [CrossRef]
- Chen, G.; Shi, L. Removal of Cd(II) and Pb(II) ions from natural water using a low-cost synthetic mineral: Behavior and mechanisms. RSC Adv. 2017, 7, 43445–43454. [Google Scholar] [CrossRef] [Green Version]
- Anwar, J.; Shafique, U.; Waheed, U.Z.; Salman, M.; Dar, A.; Anwar, S. Removal of Pb(II) and Cd(II) from water by adsorption on peels of banana. Bioresour. Technol. 2010, 101, 1752–1755. [Google Scholar] [CrossRef]
- Murray, A.; Örmeci, B. Use of polymeric sub-micron ion-exchange resins for removal of lead, copper, zinc, and nickel from natural waters. J. Environ. Sci. 2019, 75, 247–254. [Google Scholar] [CrossRef]
- Ren, S.; Meng, Z.; Sun, X.; Lu, H.; Zhang, M.; Lahori, A.H.; Bu, S. Comparison of Cd2+ adsorption onto amphoteric, amphoteric-cationic and amphoteric-anionic modified magnetic bentonites. Chemosphere 2020, 239, 124840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Wang, H.; Zhao, J. Removal of Pb(II) from aqueous solution using hydroxyapatite/calcium silicate hydrate (HAP/C-S-H) composite adsorbent prepared by a phosphate recovery process. Chem. Eng. J. 2018, 344, 53–61. [Google Scholar] [CrossRef]
- Kokkinos, E.; Simeonidis, K.; Pinakidou, F.; Katsikini, M.; Mitrakas, M. Optimization of tetravalent manganese feroxyhyte’s negative charge density: A high-performing mercury adsorbent from drinking water. Sci. Total Environ. 2017, 574, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Tresintsi, S.; Simeonidis, K.; Pliatsikas, N.; Vourlias, G.; Patsalas, P.; Mitrakas, M. The role of so 4 2 - Surface distribution in arsenic removal by iron oxy-hydroxides. J. Solid State Chem. 2014, 213, 145–151. [Google Scholar] [CrossRef]
- Tresintsi, S.; Simeonidis, K.; Vourlias, G.; Stavropoulos, G.; Mitrakas, M. Kilogram-scale synthesis of iron oxy-hydroxides with improved arsenic removal capacity: Study of Fe(II) oxidation-precipitation parameters. Water Res. 2012, 46, 5255–5267. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Kosmulski, M. Surface Charging and Points of Zero Charge; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781420051896. [Google Scholar]
- APHA Standard Methods for the Examination of Water and Wastewater, 17th ed.; Stand Methods; American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 1989.
- BSI BS EN 12457-4:2002. Characterisation of Waste. Leaching. Compliance Test for Leaching of Granular Waste Materials and Sludges, Part 2: One Stage Batch Test at Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size below 4 mm (without or with Size Reduction); European Standard: Brussels, Belgium, 2002. [Google Scholar]
- Joint Center for Powder Diffraction Studies (JCPDS). Powder Diffraction File; International Centre for Diffraction Data: Newtown Square, PA, USA, 2004. [Google Scholar]
- Pinakidou, F.; Katsikini, M.; Simeonidis, K.; Paloura, E.C.; Mitrakas, M. An X-ray absorption study of synthesis- and As adsorption-induced microstructural modifications in Fe oxy-hydroxides. J. Haz. Mat. 2015, 298, 203–209. [Google Scholar] [CrossRef]
- Tresintsi, S.; Simeonidis, K.; Mitrakas, M. Mn-feroxyhyte: The role of synthesis conditions on As(III) and As(V) removal capacity. Chem. Eng. J. 2014, 251, 192–198. [Google Scholar] [CrossRef]
- Ghiloufi, I.; El Ghoul, J.; Modwi, A.E.; Alshunaifi, I.; El Mir, L. Removal of lead (II) ion from aqueous solution using Ga-doped ZnO and Ca-doped ZnO nanopowder. Zeitschrift fur Naturforsch. Sect. A J. Phys. Sci. 2019, 74, 573–580. [Google Scholar] [CrossRef]
- Balsafar, V.M.; Pawar, A.V.; Mane, P.A. Applied Chemistry, 1st ed.; I.K. International Publishing House Pvt. Ltd.: New Delhi, India, 2010; pp. 56–57. [Google Scholar]
- Blatchley, E.R.; Thompson, J.E. Groundwater contaminants. In The Handbook of Groundwater Engineering, 1st ed.; Delleur, J.W., Ed.; CRC Press: Boca Raton, FL, USA, 1999; Volume 13, p. 10. [Google Scholar]
- Lide, D.R. CRC Handbook of Chemistry and Physics; Internet Version 2005; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 2012, 86, 119–126. [Google Scholar] [CrossRef]
- Persson, I. Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Aziz, H.A.; Adlan, M.N.; Ariffin, K.S. Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: Post treatment by high quality limestone. Bioresour. Technol. 2008, 99, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.; O’Day, P.A.; Vlassopoulos, D.; García-González, M.T.; Garrido, F. A surface complexation and ion exchange model of Pb and Cd competitive sorption on natural soils. Geochim. Cosmochim. Acta 2009, 73, 543–558. [Google Scholar] [CrossRef]







| Adsorbent | Chemical Composition | Surface Properties | Synthesis Cost | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Mn* | Ca | Na | IEP | PZC | [OH−] | [H+] | BET | ||
| (wt.%) | (mmol/g) | (m2/g) | €/kg | |||||||
| TMFx | 38.8 | 12.5 | 0.1 | 3.2 | 5.9 | 7.6 | 1.0 | 1.8 | 301 | 8 |
| TMFx-Ca | 39.0 | 12.6 | 3.3 | 0.2 | 3.6 | 7.9 | 1.4 | 2.0 | 205 | 8.5 |
| Adsorbent | pH | Q5 (μg Cd/mg) | Freundlich Parameters | Langmuir Parameters | ||||
|---|---|---|---|---|---|---|---|---|
| KF μg.mg−1(μg/L)−1/n | 1/n | R2 | KL (L·μg−1) | Qmax (μg Cd/mg) | R2 | |||
| TMFx | 7 | 3.94 | 1.50 | 0.600 | 0.991 | 0.02 | 29.10 | 0.999 |
| 8 | 5.91 | 2.47 | 0.542 | 0.993 | 0.05 | 26.76 | 0.993 | |
| TMFx-Ca | 7 | 5.88 | 2.38 | 0.561 | 0.994 | 0.05 | 26.90 | 0.994 |
| 8 | 7.29 | 3.34 | 0.485 | 0.990 | 0.09 | 23.50 | 0.991 | |
| Adsorbent | pH | Q10 (μg Pb/mg) | Freundlich Parameters | Langmuir Parameters | ||||
|---|---|---|---|---|---|---|---|---|
| KF μg·mg−1(μg/L)−1/n | 1/n | R2 | KL (L·μg−1) | Qmax (μg Pb/mg) | R2 | |||
| TMFx | 7 | 158 | 39.65 | 0.599 | 0.966 | 0.037 | 625 | 0.941 |
| 8 | 189 | 54.22 | 0.543 | 0.970 | 0.054 | 588 | 0.931 | |
| TMFx-Ca | 7 | 184 | 50.17 | 0.565 | 0.963 | 0.052 | 588 | 0.929 |
| 8 | 212 | 63.62 | 0.523 | 0.958 | 0.074 | 526 | 0.934 | |
| Metal | M2+ Ion Radius (Å) | M–O Distance of M(H2O)62+ (Å) | Hydration Free Energy (kJ/mol) | Q10 (μg/mg*) | Adsorbent Cost (€/kg of Adsorbed/Removed Metal) | ||
|---|---|---|---|---|---|---|---|
| TMFx | TFMx-Ca | TMFx | TFMx-Ca | ||||
| Cd | 0.96 | 2.30 | 1979 | 5.0 | 6.8 | 1.600 | 1.250 |
| Pb | 1.20 | 2.54 | 1450 | 28.5 | 35.0 | 280 | 243 |
| Metal | Adsorbent | Load | Residual Ca | EN 12457-01 | Threshold for Inert Wastes | |
|---|---|---|---|---|---|---|
| μg/mg | wt.% | pH | mg/kg | |||
| Cd | TMFx | 7.5 | <0.01 | 7.2 | 0.02 | 0.04 |
| TMFx-Ca | 8.5 | 0.015 | 7.2 | 0.03 | ||
| Pb | TMFx | 31.7 | <0.01 | 7.5 | 0.02 | 0.50 |
| TMFx-Ca | 38.5 | <0.01 | 7.6 | 0.01 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokkinos, E.; Chousein, C.; Simeonidis, K.; Coles, S.; Zouboulis, A.; Mitrakas, M. Improvement of Manganese Feroxyhyte’s Surface Charge with Exchangeable Ca Ions to Maximize Cd and Pb Uptake from Water. Materials 2020, 13, 1762. https://doi.org/10.3390/ma13071762
Kokkinos E, Chousein C, Simeonidis K, Coles S, Zouboulis A, Mitrakas M. Improvement of Manganese Feroxyhyte’s Surface Charge with Exchangeable Ca Ions to Maximize Cd and Pb Uptake from Water. Materials. 2020; 13(7):1762. https://doi.org/10.3390/ma13071762
Chicago/Turabian StyleKokkinos, Evgenios, Chasan Chousein, Konstantinos Simeonidis, Sandra Coles, Anastasios Zouboulis, and Manassis Mitrakas. 2020. "Improvement of Manganese Feroxyhyte’s Surface Charge with Exchangeable Ca Ions to Maximize Cd and Pb Uptake from Water" Materials 13, no. 7: 1762. https://doi.org/10.3390/ma13071762