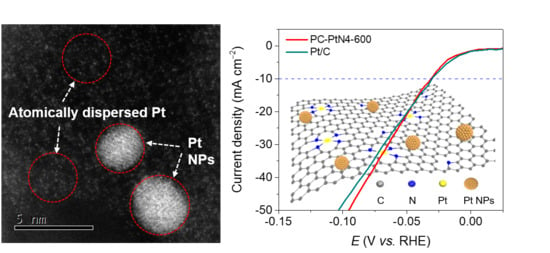
Platinum Atoms and Nanoparticles Embedded Porous Carbons for Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrocatalysts Synthesis
2.2.1. Synthesis of 5,10,15,20-tetrakis(4-bromophenyl)-21,22-dihydroporphyrin (TPP4Br)
2.2.2. Synthesis of Platinum (II) 5,10,15,20-tetrakis(4-bromophenyl)-21,22-dihydroporphyrin (Pt-TPP4Br)
2.2.3. Synthesis of Pt Porphyrin-based Conjugated Microporous Polymers (CMP-PtN4)
2.2.4. Pyrolysis of CMP-PtN4
2.3. Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pivovar, B. Catalysts for fuel cell transportation and hydrogen related uses. Nat. Catal. 2019, 2, 562. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Chen, S.; Yan, H.; Wang, C.; Wu, C.; Haleem, Y.A.; Duan, S.; Lu, J.; Ge, B.; et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat. Energy 2019, 4, 512–518. [Google Scholar] [CrossRef]
- Boddy, P. Oxygen Evolution on Semiconducting TiO. J. Electrochem. Soc. 1968, 115, 199. [Google Scholar] [CrossRef]
- Yoneyama, H.; Sakamoto, H.; Tamura, H. A Photo-electrochemical cell with production of hydrogen and oxygen by a cell reaction. Electrochim. Acta 1975, 20, 341–345. [Google Scholar] [CrossRef]
- Ohashi, K.; McCann, J.; Bockris, J.O.M. Photochemical diodes. Nature 1977, 266, 610–611. [Google Scholar] [CrossRef]
- Nozik, A.J. Stable photoelectrochemical cells for the splitting of water. Appl. Phys. Lett. 1977, 30, 567–569. [Google Scholar] [CrossRef]
- Al-Shara, N.K.; Sher, F.; Yaqoob, A.; Chen, G.Z. Electrochemical investigation of novel reference electrode Ni/Ni(OH)₂ in comparison with silver and platinum inert quasi-reference electrodes for electrolysis in eutectic molten hydroxide. Int. J. Hydrogen. Energy 2019, 44, 27224–27236. [Google Scholar] [CrossRef]
- Güleç, F.; Sher, F.; Karaduman, A. Catalytic performance of Cu- and Zr-modified beta zeolite catalysts in the methylation of 2-methylnaphthalene. Petrol. Sci. 2019, 16, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Zarren, G.; Nisar, B.; Sher, F. Synthesis of anthraquinone-based electroactive polymers: A critical review. Mater. Today Sustain. 2019, 5, 30–43. [Google Scholar] [CrossRef]
- Al-Shara, N.K.; Sher, F.; Iqbal, S.Z.; Sajid, Z.; Chen, G.Z. Electrochemical study of different membrane materials for the fabrication of stable, reproducible and reusable reference electrode. J. Energy Chem. 2020, 49, 33–41. [Google Scholar] [CrossRef]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol steam reforming for hydrogen production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, F. Hydrogen production from methane and solar energy—Process evaluations and comparison studies. Int. J. Hydrogen Energy 2014, 39, 18092–18102. [Google Scholar] [CrossRef]
- Yildiz, B.; Kazimi, M.S. Efficiency of hydrogen production systems using alternative nuclear energy technologies. Int. J. Hydrogen Energy 2006, 31, 77–92. [Google Scholar] [CrossRef]
- Sher, F.; Iqbal, S.Z.; Liu, H.; Imran, M.; Snape, C.E. Thermal and kinetic analysis of diverse biomass fuels under different reaction environment: A way forward to renewable energy sources. Energy Convers. Manag. 2020, 203, 112266. [Google Scholar] [CrossRef]
- Kim, D.; Sakimoto, K.K.; Hong, D.; Yang, P. Artificial Photosynthesis for Sustainable Fuel and Chemical Production. Angew. Chem. Int. Ed. 2015, 54, 3259–3266. [Google Scholar] [CrossRef]
- Grewe, T.; Meggouh, M.; Tüysüz, H. Nanocatalysts for Solar Water Splitting and a Perspective on Hydrogen Economy. Chem. Asian J. 2016, 11, 22–42. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The Hydrogen Issue. ChemSusChem 2011, 4, 21–36. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Lau, T.H.M.; Wu, S.; Kato, R.; Wu, T.-S.; Kulhavý, J.; Mo, J.; Zheng, J.; Foord, J.S.; Soo, Y.-L.; Suenaga, K.; et al. Engineering Monolayer 1T-MoS2 into a Bifunctional Electrocatalyst via Sonochemical Doping of Isolated Transition Metal Atoms. ACS Catal. 2019, 9, 7527–7534. [Google Scholar] [CrossRef]
- Lei, C.; Wang, Y.; Hou, Y.; Liu, P.; Yang, J.; Zhang, T.; Zhuang, X.; Chen, M.; Yang, B.; Lei, L.; et al. Efficient alkaline hydrogen evolution on atomically dispersed Ni–Nx Species anchored porous carbon with embedded Ni nanoparticles by accelerating water dissociation kinetics. Energy Environ. Sci. 2019, 12, 149–156. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Guo, X.; Chen, C.; Dong, C.-L.; Liu, R.-S.; Han, C.-P.; Li, Y.; Gogotsi, Y.; Wang, G. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Zhou, L.; Chen, C.; Han, Z.; Zhang, B.; Wu, Q.; Yang, L.; Du, L.; Bu, Y.; et al. The simplest construction of single-site catalysts by the synergism of micropore trapping and nitrogen anchoring. Nat. Commun. 2019, 10, 1657. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Chen, B.; Li, Z.; Duan, X.; Wang, L.; Lin, Y.; Yuan, T.; Zhou, F.; Hu, Y.; Yang, Z.; et al. Thermal Emitting Strategy to Synthesize Atomically Dispersed Pt Metal Sites from Bulk Pt Metal. J. Am. Chem. Soc. 2019, 141, 4505–4509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; An, P.F.; Zhou, W.; Guan, B.Y.; Zhang, P.; Dong, J.C.; Lou, X.W. Dynamic traction of lattice-confined platinum atoms into mesoporous carbon matrix for hydrogen evolution reaction. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Lun, Z.; Xia, G.; Zheng, F.; He, M.; Chen, Q. Non-precious alloy encapsulated in nitrogen-doped graphene layers derived from MOFs as an active and durable hydrogen evolution reaction catalyst. Energy Environ. Sci. 2015, 8, 3563–3571. [Google Scholar] [CrossRef]
- Gong, M.; Wang, D.-Y.; Chen, C.-C.; Hwang, B.-J.; Dai, H. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction. Nano Res. 2016, 9, 28–46. [Google Scholar] [CrossRef]
- Lu, C.; Tranca, D.; Zhang, J.; Rodri Guez Hernandez, F.N.; Su, Y.; Zhuang, X.; Zhang, F.; Seifert, G.; Feng, X. Molybdenum Carbide-Embedded Nitrogen-Doped Porous Carbon Nanosheets as Electrocatalysts for Water Splitting in Alkaline Media. ACS Nano 2017, 11, 3933–3942. [Google Scholar] [CrossRef]
- Yan, H.; Tian, C.; Wang, L.; Wu, A.; Meng, M.; Zhao, L.; Fu, H. Phosphorus-modified tungsten nitride/reduced graphene oxide as a high-performance, non-noble-metal electrocatalyst for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2015, 54, 6325–6329. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef]
- Zou, X.; Huang, X.; Goswami, A.; Silva, R.; Sathe, B.R.; Mikmekova, E.; Asefa, T. Cobalt-embedded nitrogen-rich carbon nanotubes efficiently catalyze hydrogen evolution reaction at all pH values. Angew. Chem. Int. Ed. 2014, 53, 4372–4376. [Google Scholar] [CrossRef]
- Ye, S.; Luo, F.; Zhang, Q.; Zhang, P.; Xu, T.; Wang, Q.; He, D.; Guo, L.; Zhang, Y.; He, C.; et al. Highly stable single Pt atomic sites anchored on aniline-stacked graphene for hydrogen evolution reaction. Energy Environ. Sci. 2019, 12, 1000–1007. [Google Scholar] [CrossRef]
- Lu, B.; Guo, L.; Wu, F.; Peng, Y.; Lu, J.E.; Smart, T.J.; Wang, N.; Finfrock, Y.Z.; Morris, D.; Zhang, P.; et al. Ruthenium atomically dispersed in carbon outperforms platinum toward hydrogen evolution in alkaline media. Nat. Commun. 2019, 10, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.-C.; Chan, C.-Y.; Chen, K.-T.; Tuan, H.-Y. Synthesis of popcorn-shaped gallium-platinum (GaPt3) nanoparticles as highly efficient and stable electrocatalysts for hydrogen evolution reaction. Electrochim. Acta 2019, 297, 288–296. [Google Scholar] [CrossRef]
- Lao, M.; Rui, K.; Zhao, G.; Cui, P.; Zheng, X.; Dou, S.X.; Sun, W. Platinum/Nickel Bicarbonate Heterostructures towards Accelerated Hydrogen Evolution under Alkaline Conditions. Angew. Chem. Int. Ed. 2019, 58, 5432–5437. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, S.; Xiong, L.; Wang, B.; Yang, G.; Yang, S. Surface-engineered mesoporous Pt nanodendrites with Ni dopant for highly enhanced catalytic performance in hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 12800–12807. [Google Scholar] [CrossRef]
- Kunhiraman, A.K. Hydrogen evolution reaction catalyzed by platinum nanoislands decorated on three-dimensional nanocarbon hybrid. Ionics 2019, 25, 3787–3797. [Google Scholar] [CrossRef]
- Alinezhad, A.; Gloag, L.; Benedetti, T.M.; Cheong, S.; Webster, R.F.; Roelsgaard, M.; Iversen, B.B.; Schuhmann, W.; Gooding, J.J.; Tilley, R.D. Direct Growth of Highly Strained Pt Islands on Branched Ni Nanoparticles for Improved Hydrogen Evolution Reaction Activity. J. Am. Chem. Soc. 2019, 141, 16202–16207. [Google Scholar] [CrossRef]
- Lin, L.; Sun, Z.; Yuan, M.; He, J.; Long, R.; Li, H.; Nan, C.; Sun, G.; Ma, S. Significant enhancement of the performance of hydrogen evolution reaction through shape-controlled synthesis of hierarchical dendrite-like platinum. J. Mater. Chem. A 2018, 6, 8068–8077. [Google Scholar] [CrossRef]
- Xu, G.R.; Bai, J.; Jiang, J.X.; Lee, J.M.; Chen, Y. Polyethyleneimine functionalized platinum superstructures: Enhancing hydrogen evolution performance by morphological and interfacial control. Chem. Sci. 2017, 8, 8411–8418. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Zhang, Y.; Tang, L.; Liu, C.; Gao, X.; Sun, M.; Zheng, J.; Ling, M.; Liang, C.; Lin, Z. Platinum single-atom and cluster anchored on functionalized MWCNTs with ultrahigh mass efficiency for electrocatalytic hydrogen evolution. Nano Energy 2019, 63. [Google Scholar] [CrossRef]
- Zhou, M.; Dick, J.E.; Bard, A.J. Electrodeposition of Isolated Platinum Atoms and Clusters on Bismuth-Characterization and Electrocatalysis. J. Am. Chem. Soc. 2017, 139, 17677–17682. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.K.; Liu, L.M.; et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Jiang, G.; Paul Cano, Z.; Han, L.; Yang, X.-Y.; Chen, Z. Nitrogen-doped hollow porous carbon polyhedrons embedded with highly dispersed Pt nanoparticles as a highly efficient and stable hydrogen evolution electrocatalyst. Nano Energy 2017, 40, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Browne, M.P.; Novotný, F.; Bousa, D.; Sofer, Z.; Pumera, M. Flexible Pt/Graphene Foil Containing only 6.6 wt. % of Pt has a Comparable Hydrogen Evolution Reaction Performance to Platinum Metal. ACS Sustain. Chem. Eng. 2019, 7, 11721–11727. [Google Scholar] [CrossRef]
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Zhang, F.; Zhuang, X.; Zeng, Z. New nitrogen-rich azo-bridged porphyrin conjugated microporous networks for high performance of gas capture and storage. RSC Adv. 2016, 6, 30048–30055. [Google Scholar] [CrossRef]
- Zhu, J.; Zhuang, X.; Yang, J.; Feng, X.; Hirano, S. Graphene-coupled nitrogen-enriched porous carbon nanosheets for energy storage. J. Mater. Chem. A 2017, 5, 16732–16739. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, F.; Wu, D.; Forler, N.; Liang, H.; Wagner, M.; Gehrig, D.; Hansen, M.R.; Laquai, F.; Feng, X. Two-dimensional sandwich-type, graphene-based conjugated microporous polymers. Angew. Chem. Int. Ed. 2013, 52, 9668–9672. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, C.; Lu, C.; Zhang, F.; Yuan, Z.; Zhuang, X. Two-Dimensional Porous Polymers: From Sandwich-like Structure to Layered Skeleton. Acc. Chem. Res. 2018, 51, 3191–3202. [Google Scholar] [CrossRef]
- Wang, X.; Bai, L.; Lu, J.; Zhang, X.; Liu, D.; Yang, H.; Wang, J.; Chu, P.K.; Ramakrishna, S.; Yu, X.F. Rapid Activation of Platinum with Black Phosphorus for Efficient Hydrogen Evolution. Angew. Chem. Int. Ed. 2019, 58, 19060–19066. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jia, J.; Wang, A.; Zhao, L.; Xiong, T.; Liu, H.; Zhou, W. Confined distribution of platinum clusters on MoO2 hexagonal nanosheets with oxygen vacancies as a high-efficiency electrocatalyst for hydrogen evolution reaction. Nano Energy 2019, 62, 127–135. [Google Scholar] [CrossRef]
- Lewandowska, K.; Rosiak, N.; Bogucki, A.; Cielecka-Piontek, J.; Mizera, M.; Bednarski, W.; Suchecki, M.; Szacilowski, K. Supramolecular Complexes of Graphene Oxide with Porphyrins: An Interplay between Electronic and Magnetic Properties. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Yang, J.; Xu, Z.; Wang, J.; Nuli, Y.; Zhuang, X.; Feng, X. Silicon anodes protected by a nitrogen-doped porous carbon shell for high-performance lithium-ion batteries. Nanoscale 2017, 9, 8871–8878. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, J.; Song, Y.; Wang, F. Photochemical Solid-Phase Synthesis of Platinum Single Atoms on Nitrogen-Doped Carbon with High Loading as Bifunctional Catalysts for Hydrogen Evolution and Oxygen Reduction Reactions. ACS Catal. 2018, 8, 8450–8458. [Google Scholar] [CrossRef]
- Tavakkoli, M.; Holmberg, N.; Kronberg, R.; Jiang, H.; Sainio, J.; Kauppinen, E.I.; Kallio, T.; Laasonen, K. Electrochemical Activation of Single-Walled Carbon Nanotubes with Pseudo-Atomic-Scale Platinum for the Hydrogen Evolution Reaction. ACS Catal. 2017, 7, 3121–3130. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, J.; Miao, R.; Yao, Z.; Zhuang, X.; Feng, X. Nitrogen-enriched, ordered mesoporous carbons for potential electrochemical energy storage. J. Mater. Chem. A 2016, 4, 2286–2292. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.Q.; Wu, D.X.; Chu, S.Q.; Chen, Z.Q.; Lin, Y.; Chen, M.X.; Zhang, J.; Wu, X.J.; Liang, H.W. Reversing the charge transfer between platinum and sulfur-doped carbon support for electrocatalytic hydrogen evolution. Nat. Commun. 2019, 10, 4977. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Qi, J.; Liu, M.; Zhang, S.; Fan, Q.; Liu, H.; Liu, K.; Zheng, H.; Yin, Y.; Gao, C. Aqueous Synthesis of Ultrathin Platinum/Non-Noble Metal Alloy Nanowires for Enhanced Hydrogen Evolution Activity. Angew. Chem. Int. Ed. 2018, 130, 11852–11856. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Wang, M.; Lu, C.; Ke, C.; Liu, P.; Zhu, J.; Qiu, F.; Zhuang, X. Platinum Atoms and Nanoparticles Embedded Porous Carbons for Hydrogen Evolution Reaction. Materials 2020, 13, 1513. https://doi.org/10.3390/ma13071513
Kang J, Wang M, Lu C, Ke C, Liu P, Zhu J, Qiu F, Zhuang X. Platinum Atoms and Nanoparticles Embedded Porous Carbons for Hydrogen Evolution Reaction. Materials. 2020; 13(7):1513. https://doi.org/10.3390/ma13071513
Chicago/Turabian StyleKang, Jialing, Mengjia Wang, Chenbao Lu, Changchun Ke, Pan Liu, Jinhui Zhu, Feng Qiu, and Xiaodong Zhuang. 2020. "Platinum Atoms and Nanoparticles Embedded Porous Carbons for Hydrogen Evolution Reaction" Materials 13, no. 7: 1513. https://doi.org/10.3390/ma13071513