Microfluidic-Assisted Preparation of 5-Fluorouracil-Loaded PLGA Nanoparticles as a Potential System for Colorectal Cancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
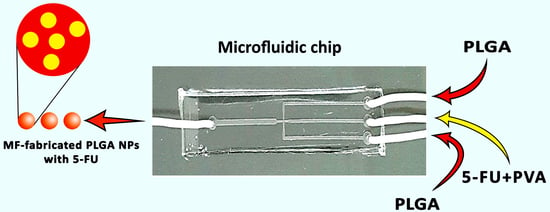
2.2. MF Chip Design
2.3. Preparation of MF-Fabricated PLGA NPs Containing 5-FU
2.4. Dynamic Light Scattering Studies
2.5. Field-Emission Scanning Electron Microscopy Studies
2.6. 5-FU Release from MF-Fabricated PLGA NPs Alongside Kinetic Models Analysis
2.7. Cell Culture
2.8. MTT Assay
2.9. Flow Cytometry
2.10. Immunofluorescence Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. The Role of Flow Rates of Polymer and Drug Solutions on PLGA/5-FU Droplets Generation
3.2. Morphology, Size and Size Distribution of NPs
3.3. 5-FU Loading and Its Release Kinetics Studies
3.4. Investigating Cytotoxicity of by MTT
3.5. Flow Cytometry and DAPI Staining
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scholfield, J.H.; Eng, C. Colorectal Cancer: Diagnosis and Clinical Management; Wiley Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Chu, E.; DeVita, V.T.; Copur, M.S. Physicians’ Cancer Chemotherapy Drug Manual; Jones & Bartlett Learning: Burlington, MA, USA, 2019; ISBN 1284144968. [Google Scholar]
- Lévi, F.; Karaboué, A.; Etienne-Grimaldi, M.C.; Paintaud, G.; Focan, C.; Innominato, P.; Chatelut, E. Pharmacokinetics of irinotecan, oxaliplatin and 5-fluorouracil during hepatic artery chronomodulated infusion: A translational european OPTILIV study. Clin. Pharm. 2017, 56, 165–177. [Google Scholar] [CrossRef]
- Talebian, J.; Wade, S.J.; Vine, K.L.; Dolatshahi Pirouz, A.; Mehrali, M.; Conde, J.; Wallace, G.G. Biopolymers for antitumor implantable drug delivery systems: Recent advances and future outlook. Adv. Mater. 2018, 30, 1706665. [Google Scholar] [CrossRef] [Green Version]
- Pardini, B.; Kumar, R.; Naccarati, A.; Novotny, J.; Prasad, R.B.; Forsti, A.; Hemminki, K.; Vodicka, P.; Bermejo, J.L. 5-Fluorouracil-based chemotherapy for colorectal cancer and MTHFR/MTRR genotypes. Br. J. Clin. Pharm. 2011, 72, 162–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjianfar, M.; Semnani, D.; Varshosaz, J. Polycaprolactone/chitosan blend nanofibers loaded by 5-fluorouracil: An approach to anticancer drug delivery system. Polym. Adv. Technol. 2018, 29, 2972–2981. [Google Scholar] [CrossRef]
- Li, L.; Li, C.; Zhou, J. Effective sustained release of 5-FU-loaded PLGA implant for improving therapeutic index of 5-FU in colon tumor. Int. J. Pharm. 2018, 550, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Leelakanok, N.; Geary, S.; Salem, A. Fabrication and use of poly (d, l-lactide-co-glycolide)-based formulations designed for modified release of 5-fluorouracil. J. Pharm. Sci. 2018, 107, 513–528. [Google Scholar] [CrossRef]
- Varshosaz, J.; Riahi, S.; Ghassami, E.; Jahanian-Najafabadi, A. Transferrin-targeted poly (butylene adipate)/terephthalate nanoparticles for targeted delivery of 5-fluorouracil in HT29 colorectal cancer cell line. J. Bioact. Compat. Polym. 2017, 32, 503–527. [Google Scholar] [CrossRef]
- Zheng, X.F.; Lian, Q.; Yang, H.; Wang, X. Surface molecularly imprinted polymer of chitosan grafted poly (methyl methacrylate) for 5-fluorouracil and controlled release. Sci. Rep. 2016, 6, 21409. [Google Scholar] [CrossRef] [Green Version]
- Shivakumar, H.G.; Fathima, S.J.; Radha, V.; Khanum, F. pH and thermosensitive 5-fluorouracil loaded poly (NIPAM-co-AAc) nanogels for cancer therapy. Rsc Adv. 2016, 6, 105495–105507. [Google Scholar]
- Rafiei, P.; Haddadi, A. Pharmacokinetic consequences of PLGA nanoparticles in Docetaxel drug delivery. Pharm. Nanotechnol. 2017, 5, 3–23. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-based nanoparticles in cancer treatment. Front. Pharm. 2018, 9, 1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashwanikumar, N.A.; Nair, S.A.; Kumar, G.V. Dual drug delivery of 5-fluorouracil (5-FU) and methotrexate (MTX) through random copolymeric nanomicelles of PLGA and polyethylenimine demonstrating enhanced cell uptake and cytotoxicity. Colloid. Surf. B 2014, 122, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Shakeri-Zadeh, A.; Khoee, S.; Shiran, M.B.; Sharifi, A.M.; Khoei, S. Synergistic effects of magnetic drug targeting using a newly developed nanocapsule and tumor irradiation by ultrasound on CT26 tumors in BALB/c mice. J. Mater. Chem. B 2015, 3, 1879–1887. [Google Scholar] [CrossRef]
- Gupta, K.K.; Pal, N.; Mishra, P.K.; Srivastava, P.; Mohanty, S.; Maiti, P. 5-Florouracil-loaded poly (lactic acid)-poly (caprolactone) hybrid scaffold: Potential chemotherapeutic implant. J. Biomed. Mater. Res. A 2014, 102, 2600–2612. [Google Scholar] [CrossRef]
- Dong, Y.-D.; Tchung, E.; Nowell, C.; Kaga, S.; Leong, N.; Mehta, D.; Kaminskas, L.M.; Boyd, B.J. Microfluidic preparation of drug-loaded PEGylated liposomes, and the impact of liposome size on tumour retention and penetration. J. Liposome Res. 2017, 27, 1–9. [Google Scholar] [CrossRef]
- Chakrabarty, K.; Zeng, J.; Zeng, J. Design Automation Methods and Tools for Microfluidics-Based Biochips; Springer: Dordrecht, The Netherlands, 2006; ISBN 1402051220. [Google Scholar]
- Martins, G.; Santos, H.A. The importance of microfluidics for the preparation of nanoparticles as advanced drug delivery systems. Expert. Opin. Drug. Del. 2018, 15, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.T.; Wereley, S.T. Fundamentals and Applications of Microfluidics; Artech house: Boston, MA, USA; London, UK, 2002; ISBN 1580533434. [Google Scholar]
- Liu, D.; Zhang, H.; Fontana, F.; Hirvonen, J.T.; Santos, H.A. Microfluidic-assisted fabrication of carriers for controlled drug delivery. Lab. Chip 2017, 17, 1856–1883. [Google Scholar] [CrossRef]
- Riahi, A.; Shaegh, S.A.; Ghaemmaghami, A.M.; Dokmeci, M.R.; Khademhosseini, A. Microfluidics for advanced drug delivery systems. Curr. Opin. Chem. Eng 2015, 7, 101–112. [Google Scholar] [CrossRef]
- Verma, S.; Gokhale, R.; Burgess, D.J. A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions. Int. J. Pharm. 2009, 380, 216–222. [Google Scholar] [CrossRef]
- Huang, K.S.; Lu, K.; Yeh, C.S.; Chung, S.R.; Lin, C.H.; Yang, C.H.; Dong, Y.S. Microfluidic controlling monodisperse microdroplet for 5-fluorouracil loaded genipin-gelatin microcapsules. J. Control. Release 2009, 137, 15–19. [Google Scholar] [CrossRef]
- Xue, P.; Wu, Y.; Menon, N.V.; Kang, Y. Microfluidic synthesis of monodisperse PEGDA microbeads for sustained release of 5-fluorouracil. Microfluid Nanofluid 2015, 18, 333–342. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Yang, F.; Gan, L.; Yang, X.; Yang, Y. Magnetic alginate microspheres detected by MRI fabricated using microfluidic technique and release behavior of encapsulated dual drugs. Int. J. Nanomed. 2017, 12, 4335. [Google Scholar] [CrossRef] [Green Version]
- Maher, S.; Santos, A.; Kumeria, T.; Kaur, G.; Lambert, M.; Forward, P.; Evdokiou, A.; Losic, D. Multifunctional microspherical magnetic and pH responsive carriers for combination anticancer therapy engineered by droplet-based microfluidics. J. Mater. Chem. B 2017, 5, 4097–4109. [Google Scholar] [CrossRef]
- Kim, P.; Kwon, K.W.; Park, M.C.; Lee, S.H.; Kim, S.M.; Suh, K.Y. Soft lithography for microfluidics: A review. Biochip J. 2008, 2, 1–11. [Google Scholar]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly (dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Eddings, M.A.; Johnson, M.A.; Gale, B.K. Determining the optimal PDMS–PDMS bonding technique for microfluidic devices. J. Micromechanics Microengineering 2008, 18, 67001. [Google Scholar] [CrossRef]
- Hwang, S.; Choi, C.H.; Lee, C.S. Regioselective surface modification of PDMS microfluidic device for the generation of monodisperse double emulsions. Macromol. Res. 2012, 20, 422–428. [Google Scholar] [CrossRef]
- Hung, L.H.; Teh, S.Y.; Jester, J.; Lee, A.P. PLGA micro/nanosphere synthesis by droplet microfluidic solvent evaporation and extraction approaches. Lab. Chip 2010, 10, 1820–1825. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Sah, E.; Sah, H. Recent trends in preparation of poly (lactide-co-glycolide) nanoparticles by mixing polymeric organic solution with antisolvent. J. Nanomater. 2015, 16, 61. [Google Scholar] [CrossRef] [Green Version]
- Dias, V.C.; Wallace, J.L.; Parsons, H.G. Modulation of cellular phospholipid fatty acids and leukotriene B4 synthesis in the human intestinal cell (CaCo-2). Gut 1992, 33, 622–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewitt, R.E.; McMarlin, A.; Kleiner, D.; Wersto, R.; Martin, P.; Tsoskas, M.; Stamp, G.W.H.; Stetler-Stevenson, W.G. Validation of a model of colon cancer progression. J. Pathol. A J. Pathol. Soc. Gt. Br. Irel. 2000, 192, 446–454. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Xu, Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed. Rep. 2015, 3, 617–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawley, T.S.; Hawley, R.G. Flow cytometry protocols. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2004; Volume 263, ISBN 1592597734. [Google Scholar]
- Weissenböck, A.; Wirth, M.; Gabor, F. WGA-grafted PLGA-nanospheres: Preparation and association with Caco-2 single cells. J. Control. Release 2004, 99, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Halayqa, M.; Domańska, U. PLGA biodegradable nanoparticles containing perphenazine or chlorpromazine hydrochloride: Effect of formulation and release. Int. J. Mol. Sci. 2014, 15, 23909–23923. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.E.; Badran, M.; Kumar, A.; Hussain, T.; Ibrahim, A.; Yassin, A.A.E.B. Physical pegylation enhances the cytotoxicity of 5-fluorouracil-loaded PLGA and PCL nanoparticles. Int. J. Nanomed. 2019, 14, 9259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawfik, E.; Ahamed, M.; Almalik, A.; Alfaqeeh, M.; Alshamsan, A. Prolonged exposure of colon cancer cells to 5-fluorouracil nanoparticles improves its anticancer activity. Saudi Pharm. J. 2017, 25, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Keyvan Rad, J.; Alinejad, Z.; Khoei, S.; Mahdavian, A.R. Controlled release and photothermal behavior of multi-purpose nanocomposite particles containing encapsulated gold-decorated magnetite and 5-FU in PLGA. Acs Biomater. Sci. Eng. 2019, 5, 4425–4434. [Google Scholar] [CrossRef]
- Xu, J.; Qi, G.; Sui, C.; Wang, W.; Sun, X. 3D h9e peptide hydrogel: An advanced three-dimensional cell culture system for anticancer prescreening of chemopreventive phenolic agents. Toxicol. Vitr. 2019, 61, 104599. [Google Scholar] [CrossRef]
- Kumari, S.; Kondapi, A.K. Lactoferrin nanoparticle mediated targeted delivery of 5-fluorouracil for enhanced therapeutic efficacy. Int. J. Biol. Macromol. 2017, 95, 232–237. [Google Scholar] [CrossRef]








| Formulation/Method | NPs Size (nm) | EE (%) | PDI | Reference |
|---|---|---|---|---|
| 5-FU loaded poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)/PLGA NPs/emulsion | ~135 | ~43 | ≤1 | [40] |
| 5-FU Loaded PLGA and PCL NPs a simple/emulsion method [without poly (ethylene glycol) (PEG)] | ~24 | ~32 | 0.137 | [41] |
| 5-FU-PLGA NPs/nanoprecipitation | ~133 | ~40 | 0.352 | [42] |
| 5-FU loaded PLGA NPs/MF technique | ~101 | ~95 | 0.083 | Current study |
| Sample | Zero-Order | First-Order | Higuchi |
|---|---|---|---|
| Neat 5-FU | 0.95 | 0.64 | 0.65 |
| MF-fabricated PLGA NPs (0.2)/5-FU | 0.63 | 0.87 | 0.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemi Toudeshkchouei, M.; Zahedi, P.; Shavandi, A. Microfluidic-Assisted Preparation of 5-Fluorouracil-Loaded PLGA Nanoparticles as a Potential System for Colorectal Cancer Therapy. Materials 2020, 13, 1483. https://doi.org/10.3390/ma13071483
Ghasemi Toudeshkchouei M, Zahedi P, Shavandi A. Microfluidic-Assisted Preparation of 5-Fluorouracil-Loaded PLGA Nanoparticles as a Potential System for Colorectal Cancer Therapy. Materials. 2020; 13(7):1483. https://doi.org/10.3390/ma13071483
Chicago/Turabian StyleGhasemi Toudeshkchouei, Mahtab, Payam Zahedi, and Amin Shavandi. 2020. "Microfluidic-Assisted Preparation of 5-Fluorouracil-Loaded PLGA Nanoparticles as a Potential System for Colorectal Cancer Therapy" Materials 13, no. 7: 1483. https://doi.org/10.3390/ma13071483