Rapid Preparation of MWCNTs/Epoxy Resin Nanocomposites by Photoinduced Frontal Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
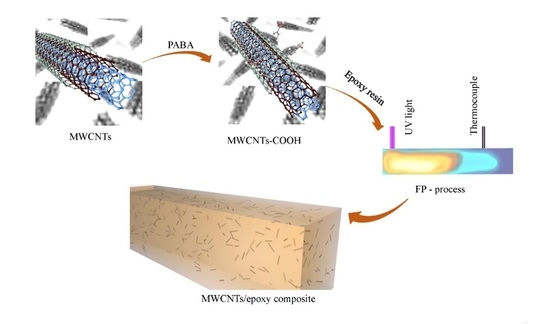
2.2. Surface Carboxylation Modification of MWCNTs
2.3. Preparation of MWCNTs/Epoxy Resin Nanocomposites Via UV-Light-Induced FP
2.4. Characterization and Measurements
3. Results
3.1. Structural Characterization
3.2. Preparation of PABA-Modified MWCNTs/Epoxy Resin Nanocomposites Via Photoinduced Frontal Polymerization
3.3. Properties of Modified MWCNTs/Epoxy Resin Nanocomposite Prepared by Photoinduced Frontal Polymerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Harik, V. Mechanics of the high aspect ratio carbon nanotubes. In Mechanics of Carbon Nanotubes; Academic Press: Cambridge, MA, USA, 2018; pp. 107–134. [Google Scholar]
- Yazdi, M.; Asl, V.H.; Pourmohammadi, M.; Roghanimamaqani, H. Mechanical properties, crystallinity, and self-nucleation of carbon nanotube-polyurethane nanocomposites. Polym. Test. 2019, 79, 106011. [Google Scholar] [CrossRef]
- Haghgoo, M.; Ansari, R.; Hassanzadeh-Aghdam, M.K. Prediction of electrical conductivity of carbon fiber-carbon nanotube-reinforced polymer hybrid composites. Compos. Part B Eng. 2019, 167, 728–735. [Google Scholar] [CrossRef]
- Zhilyaeva, M.A.; Shulga, E.V.; Shandakov, S.D.; Sergeichev, I.V.; Nasibulin, A.G. A novel straightforward wet pulling technique to fabricate carbon nanotube fibers. Carbon 2019, 15, 69–75. [Google Scholar] [CrossRef]
- Jintoku, H.; Matsuzawa, Y.; Yoshida, M. Dual use of anionic azobenzene derivative as dispersant and dopant for carbon nanotubes for enhanced thermal stability of transparent conductive films. Carbon 2019, 152, 247–254. [Google Scholar] [CrossRef]
- Pan, J.; Bian, L. A physics investigation for influence of carbon nanotube agglomeration on thermal properties of composites. Mater. Chem. Phys. 2019, 236, 121777–121786. [Google Scholar] [CrossRef]
- Marta, S.; Yu, L.; Zhao, L.; Ania, C.O.; Titiricic, M.M. Surface Modification of CNTs with N-Doped Carbon: An Effective Way of Enhancing Their Performance in Supercapacitors. ACS Sustain. Chem. Eng. 2014, 2, 1049–1055. [Google Scholar]
- Miao, J.; Dong, X.; Xu, Y.; Zhai, Z.; Zhang, L.; Ren, B.; Liu, Z. Preparation and electrochemical performance of 1,4-naphthaquinone-modified carbon nanotubes as a supercapacitor material. Org. Electron. 2019, 73, 304–310. [Google Scholar] [CrossRef]
- Huang, H.; Liu, M.; Jiang, R.; Chen, J.; Wei, Y. Fabrication and characterization of hyperbranched polyglycerol modified carbon nanotubes through the host-guest interactions. Mater. Sci. Eng. C 2018, 91, 458–465. [Google Scholar] [CrossRef]
- Lee, M.; Kwon, W.; Kwon, D.; Lee, E.; Jeong, E. Fracture toughness of the novel in-situ polytriazolesulfone modified epoxy resin for carbon fiber/epoxy composites. J. Ind. Eng. Chem. 2019, 77, 461–469. [Google Scholar] [CrossRef]
- Alshamma, F.A.; Jassim, O.A. An efficient way to produce nanocomposites of pure copper reinforced by carbon nano tubes carboxylic. J. Mater. Res. Technol. 2019, 8, 3795–3799. [Google Scholar] [CrossRef]
- Gumaste, J.L.; Singh, S.K.; Mukherjee, P.S.; Sahay, S.S.; Mishra, B.K. An efficient and inexpensive method for the production of multi-wall carbon nano tubes by the thermal plasma route. Trans. Indian Inst. Met. 2009, 62, 229–231. [Google Scholar] [CrossRef]
- Liu, Y.T.; Yao, T.T.; Zhang, W.S.; Wu, G.P. Laser welding of carbon nanotube networks on carbon fibers from ultrasonic-directed assembly. Mater. Lett. 2019, 236, 244–247. [Google Scholar] [CrossRef]
- Thi Mai Hoa, L. Characterization of multi-walled carbon nanotubes functionalized by a mixture of HNO3/H2SO4. Diam. Relat. Mater. 2018, 89, 43–51. [Google Scholar] [CrossRef]
- Shih, M.-W.; Chin, C.-J.M.; Yu, Y.-L. The role of oxygen-containing groups on the adsorption of bisphenol-A on multi-walled carbon nanotube modified by HNO3 and KOH. Process. Saf. Environ. Prot. 2017, 112, 308–314. [Google Scholar] [CrossRef]
- Zhang, P.; Kan, L.; Zhang, X.Y.; Li, R.; Qiu, C.Q.; Ma, N.; Wei, H. Super molecularly toughened and elastic epoxy resins by grafting 2-ureido-4[1H]-pyrimidone moieties on the side chain. Eur. Polym. J. 2019, 116, 126–133. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, X.; Zhang, F.; Wu, X.; Wang, X. Influence of gamma irradiation on the molecular dynamics and mechanical properties of epoxy resin. Polym. Degrad. Stab. 2019, 168, 108940–108947. [Google Scholar] [CrossRef]
- Rad, E.R.; Vahabi, H.; Anda, A.R.; Saeb, M.R.; Thomas, S. Bio-epoxy resins with inherent flame retardancy. Prog. Org. Coat. 2019, 135, 608–612. [Google Scholar] [CrossRef]
- Gibson, G. Epoxy resins. In Brydson’s Plastics Materials; Butterworth–Heinemann: Oxford, UK, 2017; pp. 773–797. [Google Scholar]
- Turk, M.; Hamerton, I.; Ivanov, D.S. Ductility potential of brittle epoxies: Thermomechanical behaviour of plastically-deformed fully-cured composite resins. Polymer 2017, 120, 43–51. [Google Scholar] [CrossRef]
- Jin, S.; Qian, L.; Qiu, Y.; Chen, Y.; Xin, F. High-efficiency flame retardant behavior of bi-DOPO compound with hydroxyl group on epoxy resin. Polym. Degrad. Stab. 2019, 166, 344–352. [Google Scholar] [CrossRef]
- Wilkinson, A.N.; Kinloch, I.A.; Othman, R.N. Low viscosity processing using hybrid CNT-coated silica particles to form electrically conductive epoxy resin composites. Polymer 2016, 98, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Sangermano, M.; D’Anna, A.; Marro, C.; Klikovits, N.; Liska, R. UV-activated frontal polymerization of glass fiber reinforced epoxy composites. Compos. Part B Eng. 2018, 143, 168–171. [Google Scholar] [CrossRef]
- Shioya, M.; Kuroyanagi, Y.; Ryu, M.; Morikawa, J. Analysis of the adhesive properties of carbon nanotube- and graphene oxide nanoribbon-dispersed aliphatic epoxy resins based on the Maxwell model. Int. J. Adhes. Adhes. 2018, 84, 27–36. [Google Scholar] [CrossRef]
- Chen, S.; Chen, L.; Wang, Y.M.; Wang, C.W.; Zhang, D.H. Preparation of nanocomposites with epoxy resins and thiol-functionalized carbon nanotubes by thiol-ene click reaction. Polym. Test. 2019, 77, 105912–105921. [Google Scholar] [CrossRef]
- Irzhak, V.I.; Dzhardimalieva, G.I.; Uflyand, I.E. Structure and properties of epoxy polymer nanocomposites reinforced with carbon nanotubes. J. Polym. Res. 2019, 26, 220–246. [Google Scholar] [CrossRef]
- Gholami, H.; Arab, H.; Mokhtarifar, M.; Maghrebi, M.; Baniadam, M. The effect of choline-based ionic liquid on CNTs’ arrangement in epoxy resin matrix. Mater. Des. 2016, 91, 180–185. [Google Scholar] [CrossRef]
- Dourani, A.; Haghgoo, M.; Hamadanian, M. Multi-walled carbon nanotube and carbon nanofiber/polyacrylonitrile aerogel scaffolds for enhanced epoxy resins. Compos. Part B Eng. 2019, 176, 107299–107308. [Google Scholar] [CrossRef]
- Yourdkhani, M.; Koohbor, B.; Lamuta, C.; Dean, L.M. Thermo-mechanical properties of thermoset polymers and composites fabricated by frontal polymerization. In Mechanics of Composite, Hybrid and Multifunctional Materials; Springer International Publishing: Cham, Switzerland, 2019; pp. 89–91. [Google Scholar]
- Jianping Zhou, S.J.; Fu, W.; Zhao, H.; Liu, Z. Curing of epoxy resin by UV-light triggered descending frontal polymerization. Chem. J. Chin. Univ. 2015, 36, 1019–1024. [Google Scholar]
- Mariani, A.; Alzari, V.; Monticelli, O.; Pojman, J.A.; Caria, G. Polymeric nanocomposites containing polyhedral oligomeric silsesquioxanes prepared via frontal polymerization. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 4514–4521. [Google Scholar] [CrossRef]
- Tsegay, N.M.; Du, X.Y.; Liu, S.S.; Wang, C.F.; Chen, s. Frontal polymerization for smart intrinsic self-healing hydrogels and its integration with microfluidics. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1412–1423. [Google Scholar] [CrossRef]
- Pomogailo, A.D.; Singh, S.; Tandon, P. Frontal polymerization of acrylamide complex with nanostructured ZnS and PbS: Their characterizations and sensing applications. Sens. Actuators B Chem. 2015, 207, 460–469. [Google Scholar]
- Zhou, J.; Jia, S.; Fu, W.; Liu, Z.; Tan, Z. Fast curing of thick components of epoxy via modified UV-triggered frontal polymerization propagating horizontally. Mater. Lett. 2016, 176, 228–231. [Google Scholar] [CrossRef]
- Goli, E.; Parikh, N.A.; Yourdkhani, M.; Hibbard, N.G.; Geubelle, P.H. Frontal polymerization of unidirectional carbon-fiber-reinforced composites. Compos. Part A Appl. Sci. Manuf. 2020, 130, 105689. [Google Scholar] [CrossRef]
- Sangermano, M.; Antonazzo, I.; Sisca, L.; Carello, M. Photoinduced cationic frontal polymerization of epoxy-carbon fibre composites. Polym. Int. 2019, 68, 1662–1665. [Google Scholar] [CrossRef] [Green Version]
- Vyas, S.; Zhang, X.; Goli, E.; Geubelle, P.H. Frontal vs. bulk polymerization of fiber-reinforced polymer-matrix composites. Compos. Sci. Technol. 2020, 198, 108303. [Google Scholar] [CrossRef]
- Goli, E.; Peterson, S.R.; Geubelle, P.H. Instabilities driven by frontal polymerization in thermosetting polymers and composites. Compos. Part B Eng. 2020, 199, 10830. [Google Scholar] [CrossRef]
- Zhu, F.; Song, Y.; Liu, S.; Nie, Y. Preparation of polymerizable thermal initiator and its application in photo-induced thermal frontal polymerization. Eur. Polym. J. 2019, 118, 107–112. [Google Scholar] [CrossRef]
- Neves, J.C.; de Castro, V.G.; Assis, A.L.S.; Veiga, A.G.; Rocco, M.L.M.; Silva, G.G. In-situ determination of amine/epoxy and carboxylic/epoxy exothermic heat of reaction on surface of modified carbon nanotubes and structural verification of covalent bond formation. Appl. Surf. Sci. 2018, 436, 495–504. [Google Scholar] [CrossRef]
- Nuvoli, D.; Alzari, V.; Nuvoli, L.; Rassu, M.; Sanna, D.; Mariani, A. Synthesis and characterization of poly(2-hydroxyethylacrylate)/beta-cyclodextrin hydrogels obtained by frontal polymerization. Carbohydr. Polym. 2016, 150, 166–171. [Google Scholar] [CrossRef]
- Li, J.; Jie, J.; Xia, J.; Li, B. Preparation of konjac glucomannan-based superabsorbent polymers by frontal polymerization. Carbohydr. Polym. 2012, 87, 757–763. [Google Scholar] [CrossRef]
- Viner, V.G.; Pojman, J.A.; Golovaty, D. The effect of phase change materials on the frontal polymerization of a triacrylate. Phys. D Nonlinear Phenom. 2010, 239, 838–847. [Google Scholar] [CrossRef]
- Rahatekar, S.S.; Koziol, K.; Butler, S.A. Optical microstructure and viscosity enhancement for an epoxy resin matrix containing multiwall carbon nanotubes. J. Rheol. 2006, 50, 599–610. [Google Scholar] [CrossRef]
- Goli, E.; Robertson, I.D.; Agarwal, H.; Pruitt, E.L.; Grolman, J.M.; Geubelle, P.H.; Moore, J.S. Frontal polymerization accelerated by continuous conductive elements. J. Appl. Polym. Sci. 2019, 136, 47418. [Google Scholar] [CrossRef]
- Goli, E.; Gai, T.; Geubelle, P.H. Impact of Boundary Heat Losses on Frontal Polymerization. J. Phys. Chem. B 2020, 124, 6404–6411. [Google Scholar] [CrossRef] [PubMed]














| Content of MWCNTs (wt %) | 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 |
| Vf of Modified MWCNTs/Epoxy (cm/min) | 0.81 | 3.15 | 3.04 | 2.72 | 2.54 | 1.43 |
| Vf of Unmodified MWCNTs/Epoxy (cm/min) | 0.81 | 2.89 | 2.63 | 2.41 | 2.15 | 0.98 |
| Content of MWCNTs (wt %) | 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 |
| Viscosity of Modified MWCNTs/Epoxy (mPa·s) | 165 | 179 | 185 | 198 | 206 | 224 |
| Viscosity of Unmodified MWCNTs/Epoxy (mPa·s) | 165 | 133 | 139 | 146 | 165 | 192 |
| Content of Modified MWCNTs (wt %) | 0% | 0.2% | 0.4% | 0.6% | 0.8% |
| Tmax (°C) | 234.5 | 227.9 | 226.6 | 224.2 | 211.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, G.; Fu, W.; Ma, Y.; Zhou, J.; Liang, H.; Kang, X.; Qi, X. Rapid Preparation of MWCNTs/Epoxy Resin Nanocomposites by Photoinduced Frontal Polymerization. Materials 2020, 13, 5838. https://doi.org/10.3390/ma13245838
Hu G, Fu W, Ma Y, Zhou J, Liang H, Kang X, Qi X. Rapid Preparation of MWCNTs/Epoxy Resin Nanocomposites by Photoinduced Frontal Polymerization. Materials. 2020; 13(24):5838. https://doi.org/10.3390/ma13245838
Chicago/Turabian StyleHu, Guofeng, Wanli Fu, Yumin Ma, Jianping Zhou, Hongbo Liang, Xinmei Kang, and Xiaolin Qi. 2020. "Rapid Preparation of MWCNTs/Epoxy Resin Nanocomposites by Photoinduced Frontal Polymerization" Materials 13, no. 24: 5838. https://doi.org/10.3390/ma13245838