The hetero-Friedel-Crafts-Bradsher Cyclizations with Formation of Ring Carbon-Heteroatom (P, S) Bonds, Leading to Organic Functional Materials
Abstract
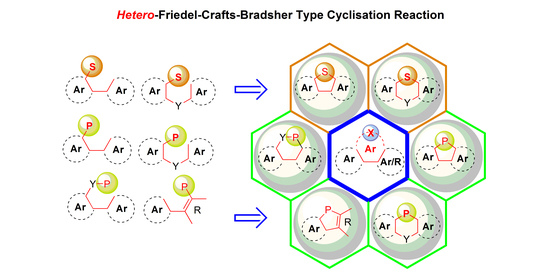
:1. Introduction
2. Cyclisation of Sulfur Derivatives
3. Cyclization of Phosphorus Derivatives
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hatakeyama, T.; Hashimoto, S.; Nakamura, M. Synthesis of Heteroatom-fused Polycyclic Aromatic Compounds via Tandem Hetero-Friedel-Crafts Reactions and Their Applications. J. Synth. Org. Chem. Jpn. 2014, 72, 1391–1397. [Google Scholar] [CrossRef]
- Hibner-Kulicka, P.; Joule, J.A.; Skalik, J.; Bałczewski, P. Recent studies of the synthesis, functionalization, optoelectronic properties and applications of dibenzophospholes. RSC Adv. 2017, 7, 9194–9236. [Google Scholar] [CrossRef] [Green Version]
- Bałczewski, P.; Kowalska, E.; Różycka-Sokołowska, E.; Skalik, J.; Owsianik, K.; Koprowski, M.; Marciniak, B.; Guziejewski, D.; Ciesielski, W. Mono-Aryl/Alkylthio-Substituted (Hetero)acenes of Exceptional Thermal and Photochemical Stability by the Thio-Friedel–Crafts/Bradsher Cyclization Reaction. Chem. Eur. J. 2019, 25, 14148–14161. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Xu, M.; Li, F.; Yu, Y. Red-Light-Controllable Liquid-Crystal Soft Actuators via Low-Power Excited Upconversion Based on Triplet–Triplet Annihilation. J. Am. Chem. Soc. 2013, 135, 16446–16453. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, D. Syntheses of Polycyclic Aromatic Compounds with Heteroatom Junctions via Tandem Hetero-Friedel-Crafts Reactions. Master′s Thesis, Kyoto University, Kyoto, Japan, 25 March 2013. [Google Scholar]
- Schöberl, A.; Wagner, A. Modern der Organischen Chemie (Houben-Weyl), 4th ed.; Müller, E., Ed.; Thieme: Stuttgart, Germany, 1955; Volume IX, pp. 217–282. [Google Scholar]
- Douglas, J.B.; Farah, B.S. Some Reactions of Methanesulfinyl Chloride. J. Org. Chem. 1958, 23, 805–807. [Google Scholar] [CrossRef]
- Gupta, S.K. New Reactions and Reagents; VI. A Simple Synthesis of N,N-Dialkylsulfonamides via the Reaction of Dialkylsulfamyl Chlorides with Aromatic Hydrocarbons. Synthesis 1977, 1, 39–41. [Google Scholar] [CrossRef]
- Yuste, F.; Linares, A.H.; Mastranzo, V.M.; Ortiz, B.; Sánchez-Obregón, R.; Fraile, A.; Ruano, J.L.G. Methyl Sulfinates as Electrophiles in Friedel–Crafts Reactions. Synthesis of Aryl Sulfoxides. J. Org. Chem. 2011, 76, 4635–4644. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Friend, R.H.; Wang, C.; Leuniger, J.; Müllen, K.J. Dibenzothienobisbenzothiophene-a novel fused-ring oligomer with high field-effect mobility. Mater. Chem. 1999, 9, 2095. [Google Scholar] [CrossRef]
- Gao, P.; Beckman, D.; Tsao, H.N.; Feng, X.; Enkelmann, V.; Pisula, W.; Müllen, K. Benzo[1,2-b:4,5-b′]bis[b]benzothiophene as solution processible organic semiconductor for field-effect transistors. Chem. Commun. 2008, 1548–1550. [Google Scholar] [CrossRef]
- Gao, P.; Beckman, D.; Tsao, H.N.; Feng, X.; Enkelmann, V.; Baumgarten, M.; Pisula, W.; Müllen, K. Dithieno[2,3-d;2′,3′-d′]benzo[1,2-b;4,5-b′]dithiophene (DTBDT) as Semiconductor for High-Performance, Solution-Processed Organic Field-Effect Transistors. Adv. Mater. 2009, 21, 213–216. [Google Scholar] [CrossRef]
- Kim, J.; Han, A.-R.; Seo, J.H.; Oh, J.H.; Yang, C. β-Alkyl substituted Dithieno[2,3-d;2′,3′-d′]benzo[1,2-b;4,5-b′]dithiophene Semiconducting Materials and Their Application to Solution-Processed Organic Transistors. Chem. Mater. 2012, 24, 3464–3472. [Google Scholar] [CrossRef]
- Li, J.; Qiao, X.; Xiong, Y.; Li, H.; Zhu, D. Five-Ring Fused Tetracyanothienoquinoids as High-Performance and Solution-Processable n-Channel Organic Semiconductors: Effect of the Branching Position of Alkyl Chains. Chem. Mater. 2014, 26, 5782–5788. [Google Scholar] [CrossRef]
- Guo, X.; Tsao, H.N.; Gao, P.; Xia, D.; An, C.; Nazeeruddin, M.K.; Baumgarten, M.; Gratzel, M.; Mullen, K. Dithieno[2,3-d;2′,3′-d′]benzo[1,2-b;4,5-b′]dithiophene based organic sensitizers for dye-sensitized solar cells. RSC Adv. 2014, 4, 54130–54133. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Baumgarten, M.; Guo, X.; Li, M.; Marszalek, T.; Alsewailem, F.D.; Pisula, W.; Müllen, K. Alkyl substituted dithienothieno[2,3-d;2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophenes as solution-processable hexathiaheptacenes. J. Mater. Chem. C 2014, 2, 3625–3630. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Feng, X.; Yang, X.; Enkelmann, V.; Baumgarten, M.; Müllen, K. Conjugated Ladder-Type Heteroacenes Bearing Pyrrole and Thiophene Ring Units: Facile Synthesis and Characterization. J. Org. Chem. 2008, 73, 9207–9213. [Google Scholar] [CrossRef]
- Gao, P.; Cho, D.; Yang, X.; Enkelmann, V.; Baumgarten, M.; Müllen, K. Heteroheptacenes with Fused Thiophene and Pyrrole Rings. Chem. Eur. J. 2010, 16, 5119–5128. [Google Scholar] [CrossRef]
- Du, C.; Ye, S.; Chen, J.; Guo, Y.; Liu, Y.; Lu, K.; Liu, Y.; Qi, T.; Gao, X.; Shuai, Z.; et al. Asymmetrical Fluorene[2,3-b]benzo[d]thiophene Derivatives: Synthesis, Solid-State Structures, and Application in Solution-Processable Organic Light-Emitting Diodes. Chem. Eur. J. 2009, 15, 8275–8282. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Luo, H.; Xie, X.; Ai, L.; Ge, Z.; Yu, G.; Liu, Y. Benzothieno[2,3-b]thiophene semiconductors: Synthesis, characterization and applications in organic field-effect transistors. J. Mater. Chem. C 2014, 2, 8804–8810. [Google Scholar] [CrossRef]
- Liu, X.; Qi, X.; Gao, J.; Zou, S.; Zhang, H.; Hao, W.; Zang, Z.; Li, H.; Hu, W. Dialkylated dibenzotetrathienoacene derivative as semiconductor for organic field effect transistors. Org. Electron. 2014, 15, 156–161. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Z. Synthesis and Properties of Naphthobisbenzothiophene Diimides. Org. Lett. 2013, 15, 1366–1369. [Google Scholar] [CrossRef]
- Zhang, S.; Qiao, X.; Chen, Y.; Wang, Y.; Edkins, R.M.; Liu, Z.; Li, H.; Fang, Q. Synthesis, Structure, and Opto-electronic Properties of Regioisomeric Pyrene–Thienoacenes. Org. Lett. 2014, 16, 342–345. [Google Scholar] [CrossRef]
- Xiong, Y.; Qiao, X.; Wu, H.; Huang, Q.; Wu, Q.; Li, J.; Gao, X.; Li, H. Syntheses and Properties of Nine-Ring-Fused Linear Thienoacenes. J. Org. Chem. 2014, 79, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Wang, Y.; Gao, J.; Liu, X.; Hao, W.; Zhang, H.; Zhang, H.; Xie, H.; Yang, C.; Li, H.; et al. Synthesis, characterization, and field-effect transistor performance of a two-dimensional starphene containing sulfur. J. Mater. Chem. C 2014, 2, 10011–10016. [Google Scholar] [CrossRef]
- Takemura, I.; Sone, R.; Nishide, H. Poly(thiaheterohelicene) derived from the long-alkylated polysulfonium precursor. Polym. Adv. Technol. 2008, 19, 1092–1096. [Google Scholar] [CrossRef]
- Oyaizu, K.; Iwasaki, T.; Tsukahara, Y.; Tsuchida, E. Linear Ladder-Type π-Conjugated Polymers Composed of Fused Thiophene Ring Systems. Macromolecules 2004, 37, 1257–1270. [Google Scholar] [CrossRef]
- Haryono, A.; Miyatake, K.; Natori, J.; Tsuchida, E. Synthesis of a Novel Oligo(p-phenylene) Ladder by Sulfide and Sulfonio Groups. Macromolecules 1999, 32, 3146–3149. [Google Scholar] [CrossRef]
- Iwasaki, T.; Katayose, K.; Kohinata, Y.; Nishide, H. A Helical Ladder Polymer: Synthesis and Magnetic Circular Dichroism of Poly[phenylene-4,6-bis(methylsulfonio)-1,3-diyl triflate]. Polym. J. 2005, 37, 592–598. [Google Scholar] [CrossRef]
- Leuninger, J.; Trimpin, S.; Räder, H.J.; Müllen, K. Novel Approach to Ladder-Type Polymers: Polydithiathianthrene via the Intramolecular Acid-Induced Cyclization of Methylsulfinyl-Substituted Poly(meta-phenylene sulfide). Macromol. Chem. Phys. 2001, 202, 2832–2842. [Google Scholar] [CrossRef]
- Miyatake, K.; Hay, A.S.; Mitsuhashi, F.; Tsuchida, E. Synthesis of Novel Ladder Polymer Electrolytes Bridged by Sulfonio and Imino Groups. Macromolecules 2001, 34, 2385–2388. [Google Scholar] [CrossRef]
- Oyaizu, K.; Missuhashi, F.; Tsuchida, E. Palladium-catalyzed synthesis of oligo(methylthio)aniline and conversion to polyacene-type electrolytes bearing phenothiazinium repeating units. Macromol. Chem. Phys. 2002, 203, 1328–1336. [Google Scholar] [CrossRef]
- Oyaizu, K.; Mikami, T.; Mitsuhashi, F.; Tsuchida, E. Synthetic Routes to Polyheteroacenes: Characterization of a Heterocyclic Ladder Polymer Containing Phenoxathiinium-type Building Blocks. Macromolecules 2002, 35, 67–78. [Google Scholar] [CrossRef]
- Tsuchida, E.; Oyaizu, K. Alkylsulfonioarylene and Thioarylene Polymers Derived from Sulfonium Electrophiles. Bull. Chem. Soc. Jpn. 2003, 76, 15–47. [Google Scholar] [CrossRef]
- Iwasaki, T.; Tsukahara, Y.; Nishide, H. Facile Preparation of Helical Ladder-type Polymers with Fused Phenoxathiine Rings. Chem. Lett. 2005, 34, 164–165. [Google Scholar] [CrossRef]
- Oyaizu, K.; Matsubara, R.; Iwasaki, T.; Tsuchida, E. Heteropolyacene with Thianthrenium Ring Systems Proving p Electron Delocalization over S Atoms. J. Macromol. Sci. A 2003, 40, 655–670. [Google Scholar] [CrossRef]
- Sun, Z.-B.; Guo, M.; Zhao, C.-H. Synthesis and Properties of Benzothieno[b]-Fused BODIPY Dyes. J. Org. Chem. 2016, 81, 229–237. [Google Scholar] [CrossRef]
- Huang, J.; Luo, H.; Wang, L.; Guo, Y.; Zhang, W.; Chen, H.; Zhu, M.; Liu, Y.; Yu, G. Dibenzoannelated Tetrathienoacene: Synthesis, Characterization, and Applications in Organic Field-Effect Transistors. Org. Lett. 2012, 14, 3300–3303. [Google Scholar] [CrossRef]
- Zheng, T.; Cai, Z.; Ho-Wu, R.; Yau, S.H.; Shaparov, V.; Goodson, T., III; Yu, L. Synthesis of Ladder-Type Thienoacenes and Their Electronic and Optical Properties. J. Am. Chem. Soc. 2016, 138, 868–875. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, J.; Hu, L.; Zhang, B.; Yang, W. Synthesis and optical and electrochemical properties of polycyclic aromatic compounds with S,S-dioxide benzothiophene fused seven rings. New J. Chem. 2015, 39, 6513–6521. [Google Scholar] [CrossRef]
- Chen, D.-M.; Wang, S.; Li, H.-X.; Zhu, X.-Z.; Zhao, C.-H. Solid-State Emissive B,S-Bridged p-Terphenyls: Synthesis, Properties, and Utility as Bifunctional Fluorescent Sensor for Hg2+ and F– Ions. Inorg. Chem. 2014, 53, 12532–12539. [Google Scholar] [CrossRef]
- Son, H.J.; Lu, L.; Chen, W.; Xu, T.; Zheng, T.; Carsten, B.; Strzalka, J.; Darling, S.B.; Chen, L.X.; Yu, L. Synthesis and Photovoltaic Effect in Dithieno[2,3-d:2′,3′-d′]Benzo[1,2-b:4,5-b′]dithiophene-Based Conjugated Polymers. Adv. Mater. 2013, 25, 838–843. [Google Scholar] [CrossRef]
- Zheng, T.; Lu, L.; Jackson, N.E.; Lou, S.J.; Chen, L.X.; Yu, L. Roles of Quinoidal Character and Regioregularity in Determining the Optoelectronic and Photovoltaic Properties of Conjugated Copolymers. Macromolecules 2014, 47, 6252–6259. [Google Scholar] [CrossRef]
- Pandya, V.B.; Jain, M.R.; Chaugule, B.V.; Patel, J.S.; Parmar, B.M.; Joshi, J.K.; Patel, P.R. Efficient Synthesis of Unsymmetrical Dibenzothiophenes by Acid-Mediated Intramolecular Cyclization of Biaryl Methyl Sulfoxides. Synth. Comm. 2012, 42, 497–505. [Google Scholar] [CrossRef]
- Du, C.; Ye, S.; Liu, Y.; Guo, Y.; Wu, T.; Liu, H.; Zheng, J.; Cheng, C.; Zhu, M.; Yua, G. Fused-seven-ring anthracene derivative with two sulfur bridges for high performance red organic light-emitting diodes. Chem. Commun. 2010, 46, 8573–8575. [Google Scholar] [CrossRef] [PubMed]
- Maier, L. Organische Phosphorverbindungen. XI. Arylierung von PSCl3 in Gegenwart von Friedel-Crafts-Aktivatoren. Ein neues Verfahren zur Darstellung von Thiophosphoäure-dihalogeniden, Thiophosphinsäurehalogeniden und tertiären Phosphinsulfiden. Helv. Chim. Acta 1964, 47, 120–132. [Google Scholar] [CrossRef]
- Olah, G.A.; Hehemann, D. Friedel-Crafts Type Preparation of Triphenylphosphine. J. Org. Chem. 1977, 42, 2190. [Google Scholar] [CrossRef]
- Diaz, A.A.; Young, J.D.; Khan, M.A.; Wehmschulte, R.J. Facile Synthesis of Unsymmetrical 9-Phospha- and 9-Arsafluorenes. Inorg. Chem. 2006, 45, 5568–5575. [Google Scholar] [CrossRef]
- Diaz, A.A.; Buster, B.; Schomisch, D.; Khan, M.A.; Baum, J.C.; Wehmschulte, R.J. Size Matters: Room Temperature P−C Bond Formation Through C−H Activation in m-Terphenyldiiodophosphines. Inorg. Chem. 2008, 47, 2858–2863. [Google Scholar] [CrossRef]
- Unoh, Y.; Yokoyama, Y.; Satoh, T.; Hirano, K.; Miura, M. Regioselective Synthesis of Benzo[b]phosphole Derivatives via Direct ortho-Alkenylation and Cyclization of Arylthiophosphinamides. Org. Lett. 2016, 18, 5436–5439. [Google Scholar] [CrossRef]
- Unoh, Y.; Satoh, T.; Hirano, K.; Miura, M. Rhodium(III)-Catalyzed Direct Coupling of Arylphosphine Derivatives with Heterobicyclic Alkenes: A Concise Route to Biarylphosphines and Dibenzophosphole Derivati. ACS Catal. 2015, 5, 6634–6639. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Hashimoto, S.; Nakamura, M. Tandem Phospha-Friedel–Crafts Reaction toward Curved π-Conjugated Frameworks with a Phosphorus Ring Junction. Org. Lett. 2011, 13, 2130–2133. [Google Scholar] [CrossRef]
- Hashimoto, S.; Nakatsuka, S.; Nakamura, M.; Hatakeyama, T. Construction of a Highly Distorted Benzene Ring in a Double Helicene. Angew. Chem. Int. Ed. 2014, 53, 14074–14076. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, S.; Gotoh, H.; Kageyama, A.; Sasada, Y.; Ikuta, T.; Hatakeyama, T. 5,9-Dioxa-13b-Oxophosphanaphtho[3,2,1-de]anthracenes Prepared by Tandem Phospha-Friedel−Crafts Reaction as Hole-/Exciton-Blocking Materials for OLEDs. Organometallics 2017, 36, 2622–2631. [Google Scholar] [CrossRef]
- Wu, B.; Santra, M.; Yoshikai, N. A Highly Modular One-Pot Multicomponent Approach to Functionalized Benzo[b]phosphole Derivatives. Angew. Chem. Int. Ed. 2014, 53, 7543–7546. [Google Scholar] [CrossRef]
- Wu, B.; Chopra, R.; Yoshikai, N. One-Pot Benzo[b]phosphole Synthesis through Sequential Alkyne Arylmagnesiation, Electrophilic Trapping, and Intramolecular Phospha-Friedel−Crafts Cyclization. Org. Lett. 2015, 17, 5666–5669. [Google Scholar] [CrossRef]
- Pastor, S.D.; Spivack, J.D.; Steinhuebel, L.P. 6H-Dibenz[c,e][1,2]oxaphosphorins: Synthesis and chemistry. Phosphorus Sulfur Silicon Relat. Elem. 1987, 31, 71–74. [Google Scholar] [CrossRef]
- Prakasha, T.K.; Day, R.O.; Holmes, R.R. New Class of Bicyclic Oxyphosphoranes with an Oxaphosphorinane Ring: Molecular Structures and Activation Energies for Ligand Exchange. J. Am. Chem. Soc. 1994, 116, 8095–8104. [Google Scholar] [CrossRef]
- Ito, T.; Iwai, T.; Nakai, T.; Mihara, M.; Mizuno, T.; Ohno, T.; Ishikawa, A.; Kobayashi, J.-I. Superacid-catalyzed Friedel–Crafts phosphination of 2-hydroxybiphenyls with phosphorus trichloride. Heteroat. Chem. 2016, 27, 336–342. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S. An overview of some recent advances in DOPO-derivatives: Chemistry and flame retardant applications. Polym. Degrad. Stabil. 2015, 113, 119–134. [Google Scholar] [CrossRef]
- Nishimura, K.; Hirano, K.; Miura, M. Synthesis of Dibenzophospholes by Tf2O-Mediated Intramolecular Phospha-Friedel−Crafts-Type Reaction. Org. Lett. 2019, 21, 1467–1470. [Google Scholar] [CrossRef]
- Nakatsuka, S.; Watanabe, Y.; Kamakura, Y.; Horike, S.; Tanaka, D.; Hatakeyama, T. Solvent-Vapor-Induced Reversible Single-Crystal-to-Single-Crystal Transformation of a Triphosphaazatriangulene-Based Metal–Organic Framework. Angew. Chem. Int. Ed. 2020, 59, 1435–1439. [Google Scholar] [CrossRef]






































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skalik, J.; Koprowski, M.; Różycka-Sokołowska, E.; Bałczewski, P. The hetero-Friedel-Crafts-Bradsher Cyclizations with Formation of Ring Carbon-Heteroatom (P, S) Bonds, Leading to Organic Functional Materials. Materials 2020, 13, 4751. https://doi.org/10.3390/ma13214751
Skalik J, Koprowski M, Różycka-Sokołowska E, Bałczewski P. The hetero-Friedel-Crafts-Bradsher Cyclizations with Formation of Ring Carbon-Heteroatom (P, S) Bonds, Leading to Organic Functional Materials. Materials. 2020; 13(21):4751. https://doi.org/10.3390/ma13214751
Chicago/Turabian StyleSkalik, Joanna, Marek Koprowski, Ewa Różycka-Sokołowska, and Piotr Bałczewski. 2020. "The hetero-Friedel-Crafts-Bradsher Cyclizations with Formation of Ring Carbon-Heteroatom (P, S) Bonds, Leading to Organic Functional Materials" Materials 13, no. 21: 4751. https://doi.org/10.3390/ma13214751