On the Anti-Corrosion Property of Dry-Gel-Conversion-Grown MFI Zeolite Coating on Aluminum Alloy
Abstract
:1. Introduction
2. Experimental
2.1. Substrate Pre-Treatment Procedure
2.2. Dry-Gel-Conversion (DGC or Steam Assisted Crystallization of Dry Precursor Film)
2.3. Characterization of Zeolite Film
2.4. Electrochemical Property Measurement
3. Results
3.1. Effect of Water Usage on the Hydrophilic Film Structure of Zeolite Coated 6061 Al Alloy
3.2. Effect of Water Usage on the Hydrophobic MFI Zeolite Coating
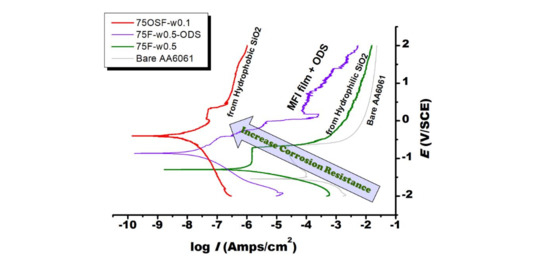
3.3. Protection of Zeolite Coated 6061 Al Alloy Substrate against Salt Corrosion
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Bagherzadeh, M.R.; Mousavinejad, T. Preparation and investigation of anticorrosion properties of the water-based epoxy-clay nanocoating modified by Na+-MMT and Cloisite 30B. Prog. Org. Coat. 2012, 74, 589–595. [Google Scholar] [CrossRef]
- Zheng, W.; Wong, S.C.; Sue, H.J. Transport behavior of PMMA/expanded graphite nanocomposites. Polymer 2002, 73, 6767–6773. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, A.; Jiang, J.; Song, D.; Chen, J.; Yang, D. Anti-corrosion performance of waterborne Zn-rich coating with modified silicon-based vehicle and lamellar Zn(Al) pigments. Prog. Nat. Sci. 2012, 22, 326–333. [Google Scholar] [CrossRef] [Green Version]
- P ajarito, B.; Kubouchi, M. Flake-Filled Polymers for Corrosion Protection. J. Chem. Eng. JPN 2013, 46, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Shchukin, D.G.; Zheludkevich, M.; Yasakau, K.; Lamaka, S.; Ferreira, M.G.S.; Möhwald, H. Layer-by-layer assembled nanocontainers for self-healing corrosion protection. Adv. Mater. 2006, 18, 1672–1678. [Google Scholar] [CrossRef]
- Radwan, A.B.; Abdullah, A.M.; Alnuaimi, N.A. Recent advances in corrosion resistant superhydrophobic coatings. Corr. Rev. 2017. [Google Scholar]
- Metroke, T.L.; Parkhill, R.L.; Knobbe, E.T. Passivation of metal alloys using sol–gel-derived materials—A review. Prog. Org. Coat. 2001, 41, 233–238. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol–gel coatings on metals for corrosion protection—Review. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Changjean, W.C.; Chiang, A.S.T.; Tsai, T.C. Anti-corrosion zeolite film by the dry-gel-conversion process. Thin Solid Films 2013, 529, 327–332. [Google Scholar] [CrossRef]
- Tsai, S.T.; ChangJeana, W.C.; Chao, P.H.; Fu, S.Y.; Tsai, T.C. Whole pH range anti-corrosion property of aluminium alloy coated with MFI zeolite film. Corr. Eng. Sci. Tech. 2018, 53 (Suppl. S1), 34–38. [Google Scholar] [CrossRef]
- Pande, H.B.; Parikh, P.A. Novel application of ZSM-5 zeolite: Corrosion-resistant coating in chemical process industry. J. Mat. Eng. Perf. 2013, 22, 190–199. [Google Scholar] [CrossRef]
- Cai, R.; Yan, Y. Corrosion-Resistant Zeolite Coatings. Corrosion 2008, 64, 271–278. [Google Scholar] [CrossRef]
- Calabrese, L. Anticorrosion behavior of zeolite coatings obtained by in situ crystallization: A critical review. Materials 2019, 12, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.Y.; Hao, Y.C.; ChangJean, W.C.; Wang, M.J.; Chiang, A.S.T.; Tsai, T.C. Growth of MFI zeolite film as corrosion protection layer of aluminum alloy. Micro. Meso. Mat. 2015, 217, 71–80. [Google Scholar] [CrossRef]
- Dias, S.A.S.; Lamaka, S.V.; Nogueira, C.A.; Diamantino, T.C.; Ferreira, M.G.S. Sol–gel coatings modified with zeolite fillers for active corrosion protection of AA2024. Corros. Sci. 2012, 62, 153–162. [Google Scholar] [CrossRef]
- Cheng, X.L.; Wang, Z.B.; Yan, Y.S. Corrosion-resistant zeolite coatings by in situ crystallization. Electrochem. Solid-State Lett. 2001, 4, B23–B26. [Google Scholar] [CrossRef]
- Beving, D.E.; McDonnell, A.M.P.; Yang, W.S.; Yan, Y.S. Corrosion resistant high-silica-zeolite MFI coating—One general solution formulation for aluminum alloy AA-2024-T3, AA-5052-H32, AA-6061-T4, and AA-7075-T6. J. Electrochem. Soc. 2006, 153, B325–B329. [Google Scholar] [CrossRef]
- Xomeritakis, G.; Gouzinis, A.; Nair, S.; Okubo, Y.; He, M.; Overney, R.M.; Tsapatsis, M. Growth, microstructure, and permeation properties of supported zeolite (MFI) “lms and membranes prepared by secondary growth. Chem. Eng. Sci. 1999, 54, 3521–3531. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Proverbio, E. Corrosion protection of aluminum 6061 in NaCl solution by silane–zeolite composite coatings. J. Coat. Technol. Res. 2012, 9, 597–607. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Yang, W.S. Fabrication of highly b-oriented MFI monolayers on various substrates. Chem. Commun. 2009, 1520–1522. [Google Scholar] [CrossRef] [PubMed]
- Tavolaro, A.; Drioli, E. Zeolite Membranes. Adv. Mater. 1999, 11, 975–996. [Google Scholar] [CrossRef]
- Chiang, A.S.T.; Chao, K.J. Membranes and films of zeolite and zeolite-like materials. J. Phys. Chem. Solids 2001, 62, 1899–1910. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M. Zeolite membranes—Recent developments and progress. Microporous Mesoporous Mater. 2008, 115, 215–233. [Google Scholar] [CrossRef]
- Sano, T.; Hasegawa, M.; Ejiri, S.; Kawakami, Y.; Yanagishita, H. Improvement of the pervaporation performance of silicalite membranes by modification with a silane coupling reagent. Microp. Mater. 1995, 5, 179–184. [Google Scholar] [CrossRef]
- Ng, E.-P.; Mintova, S. Nanoporous materials with enhanced hydrophilicity and high water sorption capacity. Microporous Mesoporous Mater. 2008, 114, 1–26. [Google Scholar] [CrossRef]
- Jiang, N.; Shang, R.; Heijman, S.G.J.; Rietveld, L.C. High-silica zeolites for adsorption of organic micro-pollutants in water treatment: A review. Water Research 2018, 144, 145–161. [Google Scholar] [CrossRef]
- Chen, N.Y. Hydrophobic properties of zeolites. J. Phys. Chem. 1976, 80, 60–64. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, H.; Jiang, J.; Wu, H.; Peng Wu, P. Hydrophobic Nanosized All-Silica Beta Zeolite: Efficient Synthesis and Adsorption Application. ACS Appl. Mater. Interfaces 2017, 9, 27273–27283. [Google Scholar] [CrossRef]
- Kosinov, N.; Sripathi, V.G.P.; Hensen, E.J.M. Improving separation performance of high-silica zeolite membranes by surface modification with triethoxyfluorosilane. Microporous Mesoporous Mater. 2014, 194, 24–30. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Beving, D.; Chen, Z.W.; Yan, Y. Molecular Sieving in a Nanoporous b-Oriented Pure-Silica-Zeolite MFI Monocrystal Film. J. Am. Chem. Soc. 2004, 126, 4122–4123. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Salazar, J.M.G.D.; Urena, A.; Manzanedo, S.; Barrena, M.I. Corrosion behaviour of AA5950 and AA6994 reinforced with Al2O3 particles in aerated 3.5% chloride solutions: Potentiodynamic measurements and microstructure evaluation. Corros. Sci. 1999, 41, 529–545. [Google Scholar] [CrossRef]
- Branzoi, V.; Golgovici, F.; Branzoi, F. Aluminium corrosion in hydrochloric acid solutions and the effect of some organic inhibitors. Mater. Chem. Phys. 2002, 78, 122–131. [Google Scholar] [CrossRef]
- Lazghab, M.; Saleh, K.; Pezron, I.; Guigon, P.; Komunjer, L. Wettability assessment of finely divided solids. Powder Technol. 2005, 157, 79–91. [Google Scholar] [CrossRef]
- Patankar, A. On the Modeling of Hydrophobic Contact Angles on Rough Surfaces. Langmuir 2003, 19, 1249–1253. [Google Scholar] [CrossRef]
- Alghunaim, A.; Newby Newby, B.M.Z. Influence of tube wettability on water contact angle of powders determined by capillary rise. Coll. Surf. A Phys. Eng. Asp. 2016, 492, 79–87. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Enhancement of the hydrophobic and anticorrosion properties of a composite zeolite coating on Al6061 substrate by modification of silane matrix. Corr. Eng. Sci. Tech. 2016, 1, 61–72. [Google Scholar]
- Matsukata, M.; Ogura, M.; Osaki, T.; Rao, P.R.H.P.; Nomura, M.; Kikuchi, E. Conversion of dry gel to microporous crystals in gas phase. Top. Catal. 1999, 9, 77–92. [Google Scholar] [CrossRef]
- Cui, M.; Wang, L.; Zhang, Y.; Wang, Y.; Meng, C. Changes of medium-range structure in the course of crystallization of mordenite from diatomite. Microporous Mesoporous Mater. 2015, 206, 52–57. [Google Scholar] [CrossRef]
- Honda, K.; Yashiki, A.; Sadakane, M.; Sano, T. Hydrothermal conversion of FAU and BEA-type zeolites into MAZ-type zeolites in the presence of non-calcined seed crystals. Microporous Mesoporous Mater. 2014, 196, 254–260. [Google Scholar] [CrossRef]








| Sample ID | Contact Angle (°) | Thickness (μm) | Ecorr (V/SCE) | Log Icorr (A/cm2) | Rp (Ω cm2) | Corrosion Rate (mpy) |
|---|---|---|---|---|---|---|
| Bare AA6061 | 73 ± 1 | 0 | −1.54 | −3.84 | 4 × 102 | 76.5 |
| 75F-w0.01 | 29 ± 1 | 4 | −1.04 | −6.73 | 2 × 105 | 9.9 × 10−2 |
| 75F-w0.1 | 27 ± 1 | 5 | −1.20 | −6.49 | 1 × 105 | 1.7 × 10−1 |
| 75F-w0.5 | <9 | 5 | −1.29 | −6.10 | 4 × 104 | 4.2 × 10−1 |
| 75F-w1.0 | <9 | 5 | −1.56 | −4.48 | 1 × 103 | 17.7 |
| 75F-w0.5-ODS | 141 ± 1 | 5 | −0.81 | −8.28 | 5 × 106 | 2.8 × 10−3 |
| 75OSF-w0.01 | 68 ± 1 | 8 | −0.59 | −7.99 | 4 × 106 | 5.4 × 10−3 |
| 75OSF-w0.1 | 56 ± 1 | 8 | −0.40 | −8.24 | 8 × 106 | 3.1 × 10−3 |
| 75OSF-w0.5 | <9 | 9 | −0.62 | −7.54 | 2 × 106 | 1.6 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, S.-T.; ChangJean, W.-C.; Huang, L.-Y.; Tsai, T.-C. On the Anti-Corrosion Property of Dry-Gel-Conversion-Grown MFI Zeolite Coating on Aluminum Alloy. Materials 2020, 13, 4595. https://doi.org/10.3390/ma13204595
Tsai S-T, ChangJean W-C, Huang L-Y, Tsai T-C. On the Anti-Corrosion Property of Dry-Gel-Conversion-Grown MFI Zeolite Coating on Aluminum Alloy. Materials. 2020; 13(20):4595. https://doi.org/10.3390/ma13204595
Chicago/Turabian StyleTsai, Shang-Tien, Wen-Chyuan ChangJean, Lin-Yi Huang, and Tseng-Chang Tsai. 2020. "On the Anti-Corrosion Property of Dry-Gel-Conversion-Grown MFI Zeolite Coating on Aluminum Alloy" Materials 13, no. 20: 4595. https://doi.org/10.3390/ma13204595