Fracture Load of CAD/CAM Feldspathic Crowns Influenced by Abutment Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Human Molars and Crown Design
2.2. Fabrication of CAD/CAM Abutments and Restorations
2.3. Embedment of Specimens
2.4. Adhesive Luting of CAD/CAM Restorations
2.5. Thermo-Mechanical Load Cycling and Fracture Loading
2.6. Statistical Analysis
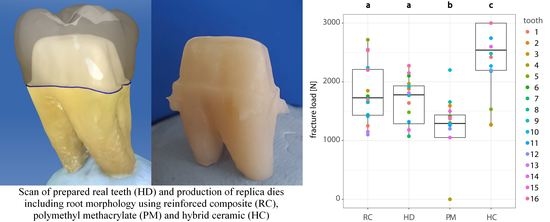
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Kelly, J.R. Dental Ceramics for Restoration and Metal Veneering. Dent. Clin. N. Am. 2017, 61, 797–819. [Google Scholar] [CrossRef] [PubMed]
- Land, M.F.; Hopp, C.D. Survival rates of all-ceramic systems differ by clinical indication and fabrication method. J. Evid. Based Dent. Pract. 2010, 10, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Denry, I.; Holloway, J. Ceramics for Dental Applications: A Review. Materials 2010, 3, 351–368. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.R.; Rungruanganunt, P.; Hunter, B.; Vailati, F. Development of a clinically validated bulk failure test for ceramic crowns. J. Prosthet. Dent. 2010, 104, 228–238. [Google Scholar] [CrossRef]
- Seydler, B.; Rues, S.; Müller, D.; Schmitter, M. In vitro fracture load of monolithic lithium disilicate ceramic molar crowns with different wall thicknesses. Clin. Oral Investig. 2014, 18, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Rosentritt, M.; Steiger, D.; Behr, M.; Handel, G.; Kolbeck, C. Influence of substructure design and spacer settings on the in vitro performance of molar zirconia crowns. J. Dent. 2009, 37, 978–983. [Google Scholar] [CrossRef]
- Agustín-Panadero, R.; Mateos-Palacios, R.; Román-Rodríguez, J.-L.; Solá-Ruíz, M.-F.; Fons-Font, A. Influence of surface preparation on fracture load of resin composite-based repairs. J. Clin. Exp. Dent. 2015, 7, e80. [Google Scholar] [CrossRef]
- Pallis, K.; Griggs, J.A.; Woody, R.D.; Guillen, G.E.; Miller, A.W. Fracture resistance of three all-ceramic restorative systems for posterior applications. J. Prosthet. Dent. 2004, 91, 561–569. [Google Scholar] [CrossRef]
- Sundh, A.; Sjögren, G. Fracture resistance of all-ceramic zirconia bridges with differing phase stabilizers and quality of sintering. Dent. Mater. 2006, 22, 778–784. [Google Scholar] [CrossRef]
- Scherrer, S.S.; de Rijk, W.G. The fracture resistance of all-ceramic crowns on supporting structures with different elastic moduli. Int. J. Prosthodont. 1993, 6, 462–467. [Google Scholar]
- Sagsoz, N.P.; Yanikoğlu, N.; Sagsoz, O. Effect of Die Materials on the Fracture Resistance of CAD/CAM Monolithic Crown Restorations. Oral Health Dent. Manag. 2016, 15, 165–168. [Google Scholar]
- Anusavice, K.J.; Tsai, Y.L. Stress Distribution in Ceramic Crown Forms as a Function of Thickness, Elastic Modulus, and Supporting Substrate. In Proceedings of the 16th Southern Biomedical Engineering Conference, Biloxi, MS, USA, 4–6 April 1997; pp. 264–267, ISBN 0-7803-3869-3. [Google Scholar]
- Bindl, A.; Lüthy, H.; Mörmann, W.H. Strength and fracture pattern of monolithic CAD/CAM-generated posterior crowns. Dent. Mater. 2006, 22, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Peutzfeldt, A.; Sahafi, A.; Flury, S. Bonding of restorative materials to dentin with various luting agents. Oper. Dent. 2011, 36, 266–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Harada, A.; Inagaki, R.; Kanno, T.; Niwano, Y.; Milleding, P.; Örtengren, U. Fracture resistance of monolithic zirconia molar crowns with reduced thickness. Acta Odontol. Scand. 2015, 73, 602–608. [Google Scholar] [CrossRef]
- Kinney, J.H.; Balooch, M.; Marshall, G.W.; Marshall, S.J. A micromechanics model of the elastic properties of human dentine. Arch. Oral Biol. 1999, 44, 813–822. [Google Scholar] [CrossRef]
- Angker, L.; Swain, M.V.; Kilpatrick, N. Micro-mechanical characterisation of the properties of primary tooth dentine. J. Dent. 2003, 31, 261–267. [Google Scholar] [CrossRef]
- Kinney, J.H.; Marshall, S.J.; Marshall, G.W. The mechanical properties of human dentin: A critical review and re-evaluation of the dental literature. Crit. Rev. Oral Biol. Med. 2003, 14, 13–29. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Du, W.; Zhou, X.-D.; Yu, H.-Y. Review of research on the mechanical properties of the human tooth. Int. J. Oral Sci. 2014, 6, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Ziskind, D.; Hasday, M.; Cohen, S.R.; Wagner, H.D. Young’s modulus of peritubular and intertubular human dentin by nano-indentation tests. J. Struct. Biol. 2011, 174, 23–30. [Google Scholar] [CrossRef]
- Kinney, J.H.; Balooch, M.; Marshall, S.J.; Marshall, G.W.; Weihs, T.P. Hardness and Young’s modulus of human peritubular and intertubular dentine. Arch. Oral Biol. 1996, 41, 9–13. [Google Scholar] [CrossRef]
- Sano, H.; Ciucchi, B.; Matthews, W.G.; Pashley, D.H. Tensile properties of mineralized and demineralized human and bovine dentin. J. Dent. Res. 1994, 73, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Meredith, N.; Sherriff, M.; Setchell, D.J.; Swanson, S.A. Measurement of the microhardness and Young’s modulus of human enamel and dentine using an indentation technique. Arch. Oral Biol. 1996, 41, 539–545. [Google Scholar] [CrossRef]
- Habelitz, S.; Marshall, G.W.; Balooch, M.; Marshall, S.J. Nanoindentation and storage of teeth. J. Biomech. 2002, 35, 995–998. [Google Scholar] [CrossRef]
- Marshall, G.W.; Habelitz, S.; Gallagher, R.; Balooch, M.; Balooch, G.; Marshall, S.J. Nanomechanical properties of hydrated carious human dentin. J. Dent. Res. 2001, 80, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, E.; Holt, A.; Swain, M.; Kilpatrick, N. The hardness and modulus of elasticity of primary molar teeth: An ultra-micro-indentation study. J. Dent. 2000, 28, 589–594. [Google Scholar] [CrossRef]
- Marshall, G.W.; Marshall, S.J.; Kinney, J.H.; Balooch, M. The dentin substrate: Structure and properties related to bonding. J. Dent. 1997, 25, 441–458. [Google Scholar] [CrossRef]
- Balooch, M.; Wu-Magidi, I.C.; Balazs, A.; Lundkvist, A.S.; Marshall, S.J.; Marshall, G.W.; Siekhaus, W.J.; Kinney, J.H. Viscoelastic properties of demineralized human dentin measured in water with atomic force microscope (AFM)-based indentation. J. Biomed. Mater. Res. 1998, 40, 539–544. [Google Scholar] [CrossRef]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, Composition and Mineralization: The role of dentin ECM in dentin formation and mineralization. Front. Biosci. (Elite Ed) 2011, 3, 711–735. [Google Scholar] [CrossRef]
- Ruse, N.D.; Sadoun, M.J. Resin-composite blocks for dental CAD/CAM applications. J. Dent. Res. 2014, 93, 1232–1234. [Google Scholar] [CrossRef] [Green Version]
- Manicone, P.F.; Rossi Iommetti, P.; Raffaelli, L. An overview of zirconia ceramics: Basic properties and clinical applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Eichberger, M.; Hoffmann, R.; Noack, F.; Schweiger, J.; Edelhoff, D.; Beuer, F. A novel CAD/CAM base metal compared to conventional CoCrMo alloys: An in-vitro study of the long-term metal-ceramic bond strength. Oral Health Dent. Manag. 2014, 13, 446–452. [Google Scholar] [PubMed]
- Fernández-Estevan, L.; Millan-Martínez, D.; Fons-Font, A.; Agustín-Panadero, R.; Román-Rodríguez, J.-L. Methodology in specimen fabrication for in vitro dental studies: Standardization of extracted tooth preparation. J. Clin. Exp. Dent. 2017, 9, e897–e900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, M.; Poggio, C.; Lasagna, A.; Chiesa, M.; Scribante, A. Vickers Micro-Hardness of New Restorative CAD/CAM Dental Materials: Evaluation and Comparison after Exposure to Acidic Drink. Materials 2019, 12, 1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, Y.S.; Oh, S.H.; Oh, W.S.; Lee, M.H.; Lee, J.J.; Bae, T.S. Effects of Liner-Bonding of Implant-Supported Glass-Ceramic Crown to Zirconia Abutment on Bond Strength and Fracture Resistance. Materials 2019, 12, 2798. [Google Scholar] [CrossRef] [Green Version]
- Yin, R.; Jang, Y.S.; Lee, M.H.; Bae, T.S. Comparative Evaluation of Mechanical Properties and Wear Ability of Five CAD/CAM Dental Blocks. Materials 2019, 12, 2252. [Google Scholar] [CrossRef] [Green Version]
- Aydın, B.; Pamir, T.; Baltaci, A.; Orman, M.N.; Turk, T. Effect of storage solutions on microhardness of crown enamel and dentin. Eur. J. Dent. 2015, 9, 262–266. [Google Scholar] [CrossRef] [Green Version]
- Goodacre, C.J.; Campagni, W.V.; Aquilino, S.A. Tooth preparations for complete crowns: An art form based on scientific principles. J. Prosthet. Dent. 2001, 85, 363–376. [Google Scholar] [CrossRef] [Green Version]
- Otto, T.; Mörmann, W.H. Clinical performance of chairside CAD/CAM feldspathic ceramic posterior shoulder crowns and endocrowns up to 12 years. Int. J. Comput. Dent. 2015, 18, 147–161. [Google Scholar]
- Venturini, A.B.; Prochnow, C.; Rambo, D.; Gundel, A.; Valandro, L.F. Effect of Hydrofluoric Acid Concentration on Resin Adhesion to a Feldspathic Ceramic. J Adhes Dent. 2015, 17, 313–320. [Google Scholar] [CrossRef]
- Krejci, I.; Reich, T.; Lutz, F.; Albertoni, M. In-vitro-Testverfahren zur Evaluation Dentaler Restaurationssysteme. 1. Computergesteuerter Kausimulator. Schweiz. Monatsschr. Zahnmed. 1990, 100, 953–960. [Google Scholar]
- RCore Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 21 May 2019).
- Wickham, H. ggplot2. Elegant Graphics for Data Analysis; Springer: Cham, Germany, 2016; ISBN 9783319242750. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Soft. 2017, 82. [Google Scholar] [CrossRef] [Green Version]
- Lenth, R. emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.2.3. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 21 May 2019).
- Zimmermann, M.; Egli, G.; Zaruba, M.; Mehl, A. Influence of material thickness on fractural strength of CAD/CAM fabricated ceramic crowns. Dent. Mater. J. 2017, 36, 778–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korostoff, E.; Pollack, S.R.; Duncanson, M.G. Viscoelastic properties of human dentin. J. Biomed. Mater. Res. 1975, 9, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Yucel, M.T.; Yondem, I.; Aykent, F.; Eraslan, O. Influence of the supporting die structures on the fracture strength of all-ceramic materials. Clin. Oral Investig. 2012, 16, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Morneburg, T.R.; Proschel, P.A. Measurement of masticatory forces and implant loads: A methodologic clinical study. Int. J. Prosthodont. 2002, 15, 20–27. [Google Scholar] [PubMed]



| Commercial Name | Material | Shade | Elastic Modulus (GPa) | Manufacturer |
|---|---|---|---|---|
| VITABLOCS Mark II | Feldspathic ceramic | 3M2C | 45 | Vita Zahnfabrik Bad Säckingen, Germany |
| VITA ENAMIC | Hybrid ceramic (HC) | 2M2HT | 30 | Vita Zahnfabrik, Bad Säckingen, Germany |
| BRILLIANT Crios | Reinforced composite (RC) | A3 | 10.3 | Coltene AG, Altstätten, Switzerland |
| Telio CAD | Polymethyl methacrylate (PM) | A3 | 3.2 | Ivoclar Vivadent, Schaan, Liechtenstein |
| - | Human dentin (HD) | - | 5–42 [4,16,17,18,19,20,21,22,23,24,25,26,27] | - |
| Preconditioning of Feldspathic Ceramic Crowns | Products |
| ultrasonic bath 2 min | (Ceramics Etch; Vita Zahnfabrik, Bad Säckingen, Germany) (Monobond Plus; Ivoclar Vivadent, Schaan, Liechtenstein) (Panavia V5; Kuraray Noritake Dental Inc., Kurashiki, Japan) (Bluephase G2; Ivoclar Vivadent, Schaan, Liechtenstein) |
| ethanol degreasing | |
| hydrofluoric acid (5%) etching 60 s, rinse 60 s, dry | |
| silanization 60 s, dry | |
| application of dual-polymerized resin cement in crown, positioning applying finger pressure, removal of excess | |
| light polymerization 5 × 40 s (occlusal, buccal, lingual, mesial and distal aspect) | |
| Preconditioning of Prepared Human Teeth | |
| cleaning | (Occlubrush; KerrHawe SA, Bioggio, Switzerland) (Panavia V5 Tooth Primer; Kuraray Noritake Dental Inc., Kurashiki, Japan) |
| application of self-etching primer 20 s, dry | |
| Preconditioning of Reinforced Composite Dies | |
| sandblasting 25–50 μm aluminum oxide, 1.5 bar | (One Coat 7 Universal; Coltene, Altstätten, Switzerland) |
| ultrasonic bath 2 min | |
| ethanol degreasing | |
| application of bond and silanization 20 s, dry | |
| Preconditioning of Hybrid Ceramic Dies | |
| ultrasonic bath 2 min | (Ceramics Etch; Vita Zahnfabrik, Bad Säckingen, Germany) (Clearfil Ceramic Primer Plus; Kuraray Noritake Dental Inc., Kurashiki, Japan) |
| ethanol degreasing | |
| hydrofluoric acid (5%) etching 60 s, rinse 60 s, dry | |
| application of bond and silanization 20 s, dry | |
| Preconditioning of Polymethyl Methacrylate Dies | |
| sandblasting 80–100 μm aluminum oxide, 1.5 bar | (SR Connect; Ivoclar Vivadent, Schaan, Liechtenstein) (Bluephase G2; Ivoclar Vivadent, Schaan, Liechtenstein) |
| ultrasonic bath 2 min | |
| ethanol degreasing | |
| silanization 3 min, dry | |
| light polymerization 40 s | |
| Group | Mean (N) | Median (N) | 95% Lower CL (N) | 95% Upper CL (N) |
|---|---|---|---|---|
| RC | 1838 | 1728 | 1565 | 2112 |
| HD | 1670 | 1778 | 1396 | 1943 |
| PM | 1142 | 1290 | 868 | 1415 |
| HC | 2435 | 2540 | 2162 | 2709 |
| Compared Groups | p-Value |
|---|---|
| RC–HD | 0.7676 |
| RC–PM | 0.0013 * |
| RC–HC | 0.0069 * |
| HD–PM | 0.0201 * |
| HD–HC | 0.0004 * |
| PM–HC | <0.0001 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bencun, M.; Ender, A.; Wiedemeier, D.B.; Mehl, A. Fracture Load of CAD/CAM Feldspathic Crowns Influenced by Abutment Material. Materials 2020, 13, 3407. https://doi.org/10.3390/ma13153407
Bencun M, Ender A, Wiedemeier DB, Mehl A. Fracture Load of CAD/CAM Feldspathic Crowns Influenced by Abutment Material. Materials. 2020; 13(15):3407. https://doi.org/10.3390/ma13153407
Chicago/Turabian StyleBencun, Mladen, Andreas Ender, Daniel B. Wiedemeier, and Albert Mehl. 2020. "Fracture Load of CAD/CAM Feldspathic Crowns Influenced by Abutment Material" Materials 13, no. 15: 3407. https://doi.org/10.3390/ma13153407