Recent Progress on Fullerene-Based Materials: Synthesis, Properties, Modifications, and Photocatalytic Applications
Abstract
:1. Introduction
2. Role of Fullerene
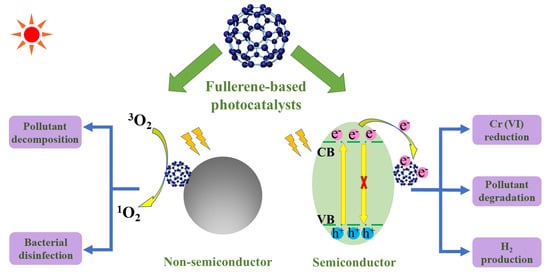
2.1. Basic Principles of Semiconducting Photocatalysis
2.2. The Role of Fullerene in Semiconductor/Fullerene Photocatalysts
3. Synthesis of Semiconductor/Fullerene Photocatalysts
3.1. Simple Adsorption Method
3.2. Hydrothermal Synthesis Method
3.3. Ball Milling Method
3.4. Other Techniques
4. The Photocatalytic Application of Fullerene/Semiconductor Photocatalysts
4.1. Fullerene Based TiO2 Photocatalysts
4.2. Metal Oxides (Except TiO2)/Fullerene Photocatalyst
4.3. Metal Sulfide/Fullerene Nanocomposites
4.4. Bismuth-Based Semiconductor/Fullerene Composites
4.5. Carbon Nitride/Fullerene Composites
4.6. Other Semiconductor/Fullerene Photocatalysts
4.7. Discussions and Conclusions for Photocatalytic Applications of Fullerene/Semiconductor Photocatalysts
5. Fullerene/Support (Non-Semiconductor) Photocatalysts for Wastewater Treatment
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Pan, Y.; Yuan, X.; Jiang, L.; Yu, H.; Zhang, J.; Wang, H.; Guan, R.; Zeng, G. Recent advances in synthesis, modification and photocatalytic applications of micro/nano-structured zinc indium sulfide. Chem. Eng. J. 2018, 354, 407–431. [Google Scholar] [CrossRef]
- Yi, H.; Qin, L.; Huang, D.; Zeng, G.; Lai, C.; Liu, X.; Li, B.; Wang, H.; Zhou, C.; Huang, F.; et al. Nano-structured bismuth tungstate with controlled morphology: Fabrication, modification, environmental application and mechanism insight. Chem. Eng. J. 2019, 358, 480–496. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Smazna, D.; Rodrigues, J.; Shree, S.; Postica, V.; Neubueser, G.; Martins, A.F.; Ben Sedrine, N.; Jena, N.K.; Siebert, L.; Schuett, F.; et al. Buckminsterfullerene hybridized zinc oxide tetrapods: Defects and charge transfer induced optical and electrical response. Nanoscale 2018, 10, 10050–10062. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Wang, Z.; Zhong, Z.; Xu, R. Highly efficient and noble metal-free NiS/CdS photocatalysts for H2 evolution from lactic acid sacrificial solution under visible light. Chem. Commun. 2010, 46, 7631–7633. [Google Scholar] [CrossRef]
- Luo, C.-Y.; Huang, W.-Q.; Xu, L.; Yang, Y.-C.; Li, X.; Hu, W.; Peng, P.; Huang, G.-F. Enhanced photocatalytic performance of an Ag3PO4 photocatalyst via fullerene modification: First-principles study. Phys. Chem. Chem. Phys. 2016, 18, 2878–2886. [Google Scholar] [CrossRef]
- Obregón, S.; Caballero, A.; Colón, G. Hydrothermal synthesis of BiVO4: Structural and morphological influence on the photocatalytic activity. Appl. Catal. B 2012, 117, 59–66. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, L.; Wang, H.; Huang, B.; Yuan, X.; Huang, J.; Zhang, J.; Zeng, G. Modulation of Bi2MoO6-Based Materials for Photocatalytic Water Splitting and Environmental Application: A Critical Review. Small 2019, 15, 1901008. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Dong, H.; Chen, X.; Leng, L.; Wu, Z.; Peng, L. In situ synthesis of In2S3@MIL-125(Ti) core-shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis. Appl. Catal. B 2016, 186, 19–29. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A reveiw. Appl. Catal. B 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic Effect of MoS2 and Graphene as Cocatalysts for Enhanced Photocatalytic H2 Production Activity of TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Han, J.; Ma, G.; Yan, H.; Wu, G.; Li, C. Photocatalytic H2 Evolution on CdS Loaded with WS2 as Cocatalyst under Visible Light Irradiation. J. Phys. Chem. C 2011, 115, 12202–12208. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Cihlář, J.; Chang, C.-Y.; Cheng, C.; Teng, H. Roles of graphene oxide in photocatalytic water splitting. Mater. Today 2013, 16, 78–84. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Y.; Park, S.-J. Recent Advances in Carbonaceous Photocatalysts with Enhanced Photocatalytic Performances: A Mini Review. Materials 2019, 12, 1916. [Google Scholar] [CrossRef] [Green Version]
- Gangu, K.K.; Maddila, S.; Jonnalagadda, S.B. A review on novel composites of MWCNTs mediated semiconducting materials as photocatalysts in water treatment. Sci. Total Environ. 2019, 646, 1398–1412. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Heng, Y.; Dai, X.; Chen, W.; Li, J. Preparation and photocatalytic ability of highly defective carbon nanotubes. J. Solid State Chem. 2009, 182, 2521–2525. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Syu, J.-M.; Cheng, C.; Chang, T.-H.; Teng, H. Graphite Oxide as a Photocatalyst for Hydrogen Production from Water. Adv. Funct. Mater. 2010, 20, 2255–2262. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Mohan, R.; Kim, S.J. Graphene oxide as a photocatalytic material. Appl. Phys. Lett. 2011, 98, 244101. [Google Scholar] [CrossRef]
- Song, L.; Guo, C.; Li, T.; Zhang, S. C-60/graphene/g-C3N4 composite photocatalyst and mutually-reinforcing synergy to improve hydrogen production in splitting water under visible light radiation. Ceram. Int. 2017, 43, 7901–7907. [Google Scholar] [CrossRef]
- Li, Q.; Xu, L.; Luo, K.W.; Huang, W.Q.; Wang, L.L.; Li, X.F.; Huang, G.F.; Yu, Y.B. Insights into enhanced visible-light photocatalytic activity of C60 modified g-C3N4 hybrids: The role of nitrogen. Phys. Chem. Chem. Phys. 2016, 18, 33094–33102. [Google Scholar] [CrossRef]
- Kumar, I.; Sharma, R.; Kumar, R.; Kumar, R.; Sharma, U. C-70 Fullerene-Catalyzed Metal-Free Photocatalytic ipso-Hydroxylation of Aryl Boronic Acids: Synthesis of Phenols. Adv. Synth. Catal. 2018, 360, 2013–2019. [Google Scholar] [CrossRef]
- Yamakoshi, Y.; Umezawa, N.; Ryu, A.; Arakane, K.; Miyata, N.; Goda, Y.; Masumizu, T.; Nagano, T. Active Oxygen Species Generated from Photoexcited Fullerene (C60) as Potential Medicines: O2-versus 1O2. J. Am. Chem. Soc. 2003, 125, 12803–12809. [Google Scholar] [CrossRef] [PubMed]
- Panahian, Y.; Arsalani, N.; Nasiri, R. Enhanced photo and sono-photo degradation of crystal violet dye in aqueous solution by 3D flower like F-TiO2(B)/fullerene under visible light. J. Photochem. Photobiol. A Chem. 2018, 365, 45–51. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, X.; Wang, X.; Wang, Y.; Zeng, Y.; Pui, D.Y.H.; Sun, J. Visible-Light Upconversion Carbon Quantum Dots Decorated TiO2 for the Photodegradation of Flowing Gaseous Acetaldehyde. Appl. Surf. Sci. 2018, 440, 266–274. [Google Scholar] [CrossRef]
- Cho, B.H.; Lee, K.B.; Miyazawa, K.I.; Ko, W.B. Preparation of Fullerene (C-60) Nanowhisker-ZnO Nanocomposites by Heat Treatment and Photocatalytic Degradation of Methylene Blue. Asian J. Chem. 2013, 25, 8027–8030. [Google Scholar] [CrossRef]
- Panagiotou, G.D.; Tzirakis, M.D.; Vakros, J.; Loukatzikou, L.; Orfanopoulos, M.; Kordulis, C.; Lycourghiotis, A. Development of 60 fullerene supported on silica catalysts for the photo-oxidation of alkenes. Appl. Catal. A Gen. 2010, 372, 16–25. [Google Scholar] [CrossRef]
- Latassa, D.; Enger, O.; Thilgen, C.; Habicher, T.; Offermanns, H.; Diederich, F. Polysiloxane-supported fullerene derivative as a new heterogeneous sensitiser for the selective photooxidation of sulfides to sulfoxides by 1O2. J. Mater. Chem. 2002, 12, 1993–1995. [Google Scholar] [CrossRef]
- Manjon, F.; Santana-Magana, M.; Garcia-Fresnadillo, D.; Orellana, G. Are silicone-supported [C60]-fullerenes an alternative to Ru(II) polypyridyls for photodynamic solar water disinfection? Photochem. Photobiol. Sci. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Guan, G.; Ye, E.; You, M.; Li, Z. Hybridized 2D Nanomaterials Toward Highly Efficient Photocatalysis for Degrading Pollutants: Current Status and Future Perspectives. Small 2020, 16, 1907087. [Google Scholar] [CrossRef]
- Perovic, K.; Dela Rosa, F.M.; Kovacic, M.; Kusic, H.; Lavrencic Stangar, U.; Fresno, F.; Dionysiou, D.D.; Bozic, A.L. Recent Achievements in Development of TiO2-Based Composite Photocatalytic Materials for Solar Driven Water Purification and Water Splitting. Materials 2020, 13, 1338. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Zhu, R.; Zhu, J.; Liang, X.; Zhu, G.; Liu, Y.; Xu, Y.; He, H. Fullerene modification of Ag3PO4 for the visible-light-driven degradation of acid red 18. RSC Adv. 2016, 6, 85962–85969. [Google Scholar] [CrossRef]
- Sepahvand, S.; Farhadi, S. Preparation and characterization of fullerene (C-60)-modified BiVO4/Fe3O4 nanocomposite by hydrothermal method and study of its visible light photocatalytic and catalytic activity. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 417–432. [Google Scholar] [CrossRef]
- Virovska, D.; Paneva, D.; Manolova, N.; Rashkov, I.; Karashanova, D. Photocatalytic self-cleaning poly(L-lactide) materials based on a hybrid between nanosized zinc oxide and expanded graphite or fullerene. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-S.; Huang, W.-Q.; Zhou, B.-X.; Peng, P.; Hu, W.-Y.; Long, M.-Q.; Huang, G.-F. The mechanism of enhanced photocatalytic activity of SnO2 through fullerene modification. Curr. Appl. Phys. 2017, 17, 1547–1556. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Wang, Y.; Yao, W.; Zhu, Y. Enhanced oxidation ability of g-C3N4 photocatalyst via C60 modification. Appl. Catal. B 2014, 153, 262–270. [Google Scholar] [CrossRef]
- Meng, Z.-D.; Zhu, L.; Oh, W.-C. Preparation and high visible-light-induced photocatalytic activity of CdSe and CdSe-C-60 nanoparticles. J. Ind. Eng. Chem. 2012, 18, 2004–2009. [Google Scholar] [CrossRef]
- Luo, C.-Y.; Huang, W.-Q.; Hu, W.; Peng, P.; Huang, G.-F. Non-covalent functionalization of WS2 monolayer with small fullerenes: Tuning electronic properties and photoactivity. Dalton Trans. 2016, 45, 13383–13391. [Google Scholar] [CrossRef]
- Ju, L.; Wu, P.; Lai, X.; Yang, S.; Gong, B.; Chen, M.; Zhu, N. Synthesis and characterization of Fullerene modified ZnAlTi-LDO in photo-degradation of Bisphenol A under simulated visible light irradiation. Environ. Pollut. 2017, 228, 234–244. [Google Scholar] [CrossRef]
- Ma, D.; Zhong, J.; Li, J.; Wang, L.; Peng, R. Enhanced photocatalytic activity of BiOCl by C-70 modification and mechanism insight. Appl. Surf. Sci. 2018, 443, 497–505. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, T.; Fu, H.; Zhao, J.; Zhu, Y. Synergetic effect of Bi2WO6 photocatalyst with C-60 and enhanced photoactivity under visible irradiation. Environ. Sci. Technol. 2007, 41, 6234–6239. [Google Scholar] [CrossRef]
- Aich, N.; Flora, J.; Saleh, N. Preparation and characterization of stable aqueous higher-order fullerenes. Nanotechnology 2012, 23, 55705. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Bacsa, R.R.; Benyounes, A.; Axet, R.; Serp, P.; Silva, C.G.; Silva, A.M.T.; Faria, J.L. Synergistic effect between carbon nanomaterials and ZnO for photocatalytic water decontamination. J. Catal. 2015, 331, 172–180. [Google Scholar] [CrossRef]
- Cai, Q.; Hu, Z.; Zhang, Q.; Li, B.; Shen, Z. Fullerene (C-60)/CdS nanocomposite with enhanced photocatalytic activity and stability. Appl. Surf. Sci. 2017, 403, 151–158. [Google Scholar] [CrossRef]
- Prylutskyy, Y.I.; Petrenko, V.I.; Ivankov, O.I.; Kyzyma, O.A.; Bulavin, L.A.; Litsis, O.O.; Evstigneev, M.P.; Cherepanov, V.V.; Naumovets, A.G.; Ritter, U. On the Origin of C60 Fullerene Solubility in Aqueous Solution. Langmuir 2014, 30, 3967–3970. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Liao, X.; Song, F.; Zhou, H. Fullerene modified C3N4 composites with enhanced photocatalytic activity under visible light irradiation. Dalton Trans. 2014, 43, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, H.; Shen, Y.; Qu, J. Photocatalytic reduction of bromate at C60 modified Bi2MoO6 under visible light irradiation. Appl. Catal. B 2011, 106, 63–68. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Xu, T.; Zhu, S.; Zhu, Y. Surface hybridization effect of C60 molecules on TiO2 and enhancement of the photocatalytic activity. J. Mol. Catal. A Chem. 2010, 331, 7–14. [Google Scholar] [CrossRef]
- Yu, J.; Ma, T.; Liu, G.; Cheng, B. Enhanced photocatalytic activity of bimodal mesoporous titania powders by C-60 modification. Dalton Trans. 2011, 40, 6635–6644. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, K.; Dai, K.; Walker, S.L.; Huang, Q.; Yin, X.; Cai, P. Efficient Photocatalytic Disinfection of Escherichia coli O157:H7 using C-70-TiO2 Hybrid under Visible Light Irradiation. Sci. Rep. 2016, 6, 25702. [Google Scholar] [CrossRef] [Green Version]
- Dai, K.; Yao, Y.; Liu, H.; Mohamed, I.; Chen, H.; Huang, Q. Enhancing the photocatalytic activity of lead molybdate by modifying with fullerene. J. Mol. Catal. A Chem. 2013, 374, 111–117. [Google Scholar] [CrossRef]
- Lin, X.; Xi, Y.; Zhao, R.; Shi, J.; Yan, N. Construction of C-60-decorated SWCNTs (C-60-CNTs)/bismuth-based oxide ternary heterostructures with enhanced photocatalytic activity. RSC Adv. 2017, 7, 53847–53854. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.-D.; Zhu, L.; Ullah, K.; Ye, S.; Sun, Q.; Jang, W.K.; Oh, W.-C. Study of the photochemically generated of oxygen species by fullerene photosensitized CoS2 nanocompounds. Mater. Res. Bull. 2014, 49, 272–278. [Google Scholar] [CrossRef]
- Tahir, M.B.; Nabi, G.; Rafique, M.; Khalid, N.R. Role of fullerene to improve the WO3 performance for photocatalytic applications and hydrogen evolution. Int. J. Energy Res. 2018, 42, 4783–4789. [Google Scholar] [CrossRef]
- Guan, J.; Wu, J.; Jiang, D.; Zhu, X.; Guan, R.; Lei, X.; Du, P.; Zeng, H.; Yang, S. Hybridizing MoS2 and C-60 via a van der Waals heterostructure toward synergistically enhanced visible light photocatalytic hydrogen production activity. Int. J. Hydrog. Energy 2018, 43, 8698–8706. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Guan, J.; Zhen, J.; Sun, Z.; Du, P.; Lu, Y.; Yang, S. A facile mechanochemical route to a covalently bonded graphitic carbon nitride (g-C3N4) and fullerene hybrid toward enhanced visible light photocatalytic hydrogen production. Nanoscale 2017, 9, 5615–5623. [Google Scholar] [CrossRef]
- Meng, Z.-D.; Peng, M.-M.; Zhu, L.; Oh, W.-C.; Zhang, F.-J. Fullerene modification CdS/TiO2 to enhancement surface area and modification of photocatalytic activity under visible light. Appl. Catal. B Environ. 2012, 113, 141–149. [Google Scholar] [CrossRef]
- Li, J.; Ko, W.B. Facile Synthesis of MoS2-C60Nanocomposites and Their Application to Catalytic Reduction and Photocatalytic Degradation. Elastom. Compos. 2016, 51, 286–300. [Google Scholar] [CrossRef]
- Apostolopoulou, V.; Vakros, J.; Kordulis, C.; Lycourghiotis, A. Preparation and characterization of 60 fullerene nanoparticles supported on titania used as a photocatalyst. Colloids Surf. A Physicochem. Eng. Asp. 2009, 349, 189–194. [Google Scholar] [CrossRef]
- Hamandi, M.; Berhault, G.; Dappozze, F.; Guillard, C.; Kochkar, H. Titanium dioxide nanotubes/polyhydroxyfullerene composites for formic acid photodegradation. Appl. Surf. Sci. 2017, 412, 306–318. [Google Scholar] [CrossRef]
- Donar, Y.O.; Bilge, S.; Sinag, A.; Pliekhov, O. TiO2/Carbon Materials Derived from Hydrothermal Carbonization of Waste Biomass: A Highly Efficient, Low-Cost Visible-Light-Driven Photocatalyst. Chemcatchem 2018, 10, 1134–1139. [Google Scholar] [CrossRef]
- Mu, S.; Long, Y.; Kang, S.-Z.; Mu, J. Surface modification of TiO2 nanoparticles with a C60 derivative and enhanced photocatalytic activity for the reduction of aqueous Cr(VI) ions. Catal. Commun. 2010, 11, 741–744. [Google Scholar] [CrossRef]
- Zhao, Z.; An, H.; Lin, J.; Feng, M.; Murugadoss, V.; Ding, T.; Liu, H.; Shao, Q.; Mai, X.; Wang, N.; et al. Progress on the Photocatalytic Reduction Removal of Chromium Contamination. Chem. Rec. 2019, 19, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.-F.; Qian, H.; Mele, G.; De Riccardis, A.; Zhao, R.; Chen, J.; Wu, H.; Hu, N.-J. Impact of different TiO2 samples and porphyrin substituents on the photocatalytic performance of TiO2@copper porphyrin composites. Catal. Today 2017, 281, 45–52. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Youssef, Z.; Colombeau, L.; Yesmurzayeva, N.; Baros, F.; Vanderesse, R.; Hamieh, T.; Toufaily, J.; Frochot, C.; Roques-Carmes, T.; Acherar, S. Dye-sensitized nanoparticles for heterogeneous photocatalysis: Cases studies with TiO2, ZnO, fullerene and graphene for water purification. Dyes Pigm. 2018, 159, 49–71. [Google Scholar] [CrossRef]
- Oh, W.-C.; Jung, A.-R.; Ko, W.-B. Preparation of fullerene/TiO2 composite and its photocatalytic effect. J. Ind. Eng. Chem. 2007, 13, 1208–1214. [Google Scholar]
- Zhang, X.; Wang, Q.; Zou, L.-H.; You, J.-W. Facile fabrication of titanium dioxide/fullerene nanocomposite and its enhanced visible photocatalytic activity. J. Colloid Interface Sci. 2016, 466, 56–61. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Dai, K.; Cai, P.; Chen, H.; Yang, C.; Huang, Q. Fullerene C-70-TiO2 hybrids with enhanced photocatalytic activity under visible light irradiation. J. Mater. Chem. A 2015, 3, 21090–21098. [Google Scholar] [CrossRef]
- Fu, H.; Xu, T.; Zhu, S.; Zhu, Y. Photocorrosion Inhibition and Enhancement of Photocatalytic Activity for ZnO via Hybridization with C-60. Environ. Sci. Technol. 2008, 42, 8064–8069. [Google Scholar] [CrossRef]
- Behera, A.; Mansingh, S.; Das, K.K.; Parida, K. Synergistic ZnFe2O4-carbon allotropes nanocomposite photocatalyst for norfloxacin degradation and Cr (VI) reduction. J. Colloid Interface Sci. 2019, 544, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yang, J.; Chen, Q.; Zhang, S. Enhanced photocatalytic activity of Ag3PO4 via Fullerene C-60 modification. Appl. Organomet. Chem. 2018, 32, e4472. [Google Scholar] [CrossRef]
- Li, G.; Jiang, B.; Li, X.; Lian, Z.; Xiao, S.; Zhu, J.; Zhang, D.; Li, H. C-60/Bi2TiO4F2 Heterojunction Photocatalysts with Enhanced Visible-Light Activity for Environmental Remediation. ACS Appl. Mater. Interface 2013, 5, 7190–7197. [Google Scholar] [CrossRef] [PubMed]
- Sepahvand, S.; Farhadi, S. Fullerene-modified magnetic silver phosphate (Ag3PO4/Fe3O4/C-60) nanocomposites: Hydrothermal synthesis, characterization and study of photocatalytic, catalytic and antibacterial activities. RSC Adv. 2018, 8, 10124–10140. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.-D.; Zhu, L.; Choi, J.-G.; Chen, M.-L.; Oh, W.-C. Effect of Pt treated fullerene/TiO2 on the photocatalytic degradation of MO under visible light. J. Mater. Chem. 2011, 21, 7596–7603. [Google Scholar] [CrossRef]
- Meng, Z.-D.; Zhang, F.-J.; Zhu, L.; Park, C.-Y.; Ghosh, T.; Choi, J.-G.; Oh, W.-C. Synthesis and characterization of M-fullerene/TiO2 photocatalysts designed for degradation azo dye. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 2175–2182. [Google Scholar] [CrossRef]
- Islam, M.T.; Hangkun, J.; Ting, Y.; Zubia, E.; Goos, A.G.; Bernal, R.A.; Botez, C.E.; Narayan, M.; Chan, C.K.; Noveron, J.C. Fullerene stabilized gold nanoparticles supported on titanium dioxide for enhanced photocatalytic degradation of methyl orange and catalytic reduction of 4-nitrophenol. J. Environ. Chem. Eng. 2018, 6, 3827–3836. [Google Scholar] [CrossRef]
- Meng, Z.-D.; Zhu, L.; Choi, J.-G.; Park, C.-Y.; Oh, W.-C. Preparation, characterization and photocatalytic behavior of WO3-fullerene/TiO2 catalysts under visible light. Nanoscale Res. Lett. 2011, 6, 459. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.M.; Sandhya, K.Y. Visible light responsive titanium dioxide-cyclodextrin-fullerene composite with reduced charge recombination and enhanced photocatalytic activity. Carbon 2014, 70, 249–257. [Google Scholar] [CrossRef]
- Bai, W.; Krishna, V.; Wang, J.; Moudgil, B.; Koopman, B. Enhancement of nano titanium dioxide photocatalysis in transparent coatings by polyhydroxy fullerene. Appl. Catal. B Environ. 2012, 125, 128–135. [Google Scholar] [CrossRef]
- Lim, J.; Monllor-Satoca, D.; Jang, J.S.; Lee, S.; Choi, W. Visible light photocatalysis of fullerol-complexed TiO2 enhanced by Nb doping. Appl. Catal. B 2014, 153, 233–240. [Google Scholar] [CrossRef]
- Song, T.; Huo, J.; Liao, T.; Zeng, J.; Qin, J.; Zeng, H. Fullerene C-60 modified Cr2-xFexO3 nanocomposites for enhanced photocatalytic activity under visible light irradiation. Chem. Eng. J. 2016, 287, 359–366. [Google Scholar] [CrossRef]
- Song, T.; Zhang, P.; Zeng, J.; Wang, T.; Ali, A.; Zeng, H. Boosting the photocatalytic H-2 evolution activity of Fe2O3 polymorphs (alpha-, gamma- and beta-Fe2O3) by fullerene C-60 -modification and dye-sensitization under visible light irradiation. RSC Adv. 2017, 7, 29184–29192. [Google Scholar] [CrossRef] [Green Version]
- Lian, Z.; Xu, P.; Wang, W.; Zhang, D.; Xiao, S.; Li, X.; Li, G. C-60-Decorated CdS/TiO2 Mesoporous Architectures with Enhanced Photostability and Photocatalytic Activity for H-2 Evolution. ACS Appl. Mater. Interface 2015, 7, 4533–4540. [Google Scholar] [CrossRef]
- Chai, B.; Peng, T.; Zhang, X.; Mao, J.; Li, K.; Zhang, X. Synthesis of C-60-decorated SWCNTs (C-60-d-CNTs) and its TiO2-based nanocomposite with enhanced photocatalytic activity for hydrogen production. Dalton Trans. 2013, 42, 3402–3409. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Zeng, H. A novel triphenylamine functionalized bithiazole-metal complex with C-60 for photocatalytic hydrogen production under visible light irradiation. J. Mater. Chem. A 2015, 3, 6258–6264. [Google Scholar] [CrossRef]
- Shahzad, K.; Tahir, M.B.; Sagir, M. Engineering the performance of heterogeneous WO3/fullerene@Ni3B/Ni(OH)2 Photocatalysts for Hydrogen Generation. Int. J. Hydrog. Energy 2019, 44, 21738–21745. [Google Scholar] [CrossRef]
- Katsumata, K.-I.; Matsushita, N.; Okada, K. Preparation of TiO2-Fullerene Composites and Their Photocatalytic Activity under Visible Light. Int. J. Photoenergy 2012. [Google Scholar] [CrossRef] [Green Version]
- Grandcolas, M.; Ye, J.; Miyazawa, K. Titania nanotubes and fullerenes C-60 assemblies and their photocatalytic activity under visible light. Ceram. Int. 2014, 40, 1297–1302. [Google Scholar] [CrossRef]
- Bastakoti, B.P.; Ishihara, S.; Leo, S.-Y.; Ariga, K.; Wu, K.C.W.; Yamauchi, Y. Polymeric Micelle Assembly for Preparation of Large-Sized Mesoporous Metal Oxides with Various Compositions. Langmuir 2014, 30, 651–659. [Google Scholar] [CrossRef]
- Moor, K.J.; Valle, D.C.; Li, C.; Kim, J.-H. Improving the Visible Light Photoactivity of Supported Fullerene Photocatalysts through the Use of C-70 Fullerene. Environ. Sci. Technol. 2015, 49, 6190–6197. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gleissner, E.H.; Tiu, E.G.; Yamakoshi, Y. C70 as a Photocatalyst for Oxidation of Secondary Benzylamines to Imines. Org. Lett. 2016, 18, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Arbogast, J.W.; Foote, C.S. Photophysical properties of C70. J. Am. Chem. Soc. 1991, 113, 8886–8889. [Google Scholar] [CrossRef]
- Cho, E.-C.; Ciou, J.-H.; Zheng, J.-H.; Pan, J.; Hsiao, Y.-S.; Lee, K.-C.; Huang, J.-H. Fullerene C-70 decorated TiO2 nanowires for visible-light-responsive photocatalyst. Appl. Surf. Sci. 2015, 355, 536–546. [Google Scholar] [CrossRef]
- Oh, W.-C.; Ko, W.-B. Characterization and photonic properties for the Pt-fullerene/TiO2 composites derived from titanium (IV) n-butoxide and C-60. J. Ind. Eng. Chem. 2009, 15, 791–797. [Google Scholar] [CrossRef]
- Kanchanatip, E.; Grisdanurak, N.; Thongruang, R.; Neramittagapong, A. Degradation of paraquat under visible light over fullerene modified V-TiO2. React. Kinet. Mech. Catal. 2011, 103, 227–237. [Google Scholar] [CrossRef]
- Oh, W.-C.; Zhang, F.-J.; Chen, M.-L. Synthesis and characterization of V-C-60/TiO2 photocatalysts designed for degradation of methylene blue. J. Ind. Eng. Chem. 2010, 16, 299–304. [Google Scholar] [CrossRef]
- Meng, Z.-D.; Zhu, L.; Ye, S.; Sun, Q.; Ullah, K.; Cho, K.-Y.; Oh, W.-C. Fullerene modification CdSe/TiO2 and modification of photocatalytic activity under visible light. Nanoscale Res. Lett. 2013, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pickering, K.D.; Wiesner, M.R. Fullerol-sensitized production of reactive oxygen species in aqueous solution. Environ. Sci. Technol. 2005, 39, 1359–1365. [Google Scholar] [CrossRef]
- Schreiner, K.M.; Filley, T.R.; Blanchette, R.A.; Bowen, B.B.; Bolskar, R.D.; Hockaday, W.C.; Masiello, C.A.; Raebiger, J.W. White-Rot Basidiomycete-Mediated Decomposition of C60 Fullerol. Environ. Sci. Technol. 2009, 43, 3162–3168. [Google Scholar] [CrossRef] [Green Version]
- Vileno, B.; Marcoux, P.R.; Lekka, M.; Sienkiewicz, A.; Fehér, T.; Forró, L. Spectroscopic and Photophysical Properties of a Highly Derivatized C60 Fullerol. Adv. Funct. Mater. 2006, 16, 120–128. [Google Scholar] [CrossRef]
- Husebo, L.O.; Sitharaman, B.; Furukawa, K.; Kato, T.; Wilson, L.J. Fullerenols revisited as stable radical anions. J. Am. Chem. Soc. 2004, 126, 12055–12064. [Google Scholar] [CrossRef] [PubMed]
- Trajković, S.; Dobrić, S.; Jaćević, V.; Dragojević-Simić, V.; Milovanović, Z.; Đorđević, A. Tissue-protective effects of fullerenol C60(OH)24 and amifostine in irradiated rats. Colloids Surf. B Biointerfaces 2007, 58, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Krishna, V.; Noguchi, N.; Koopman, B.; Moudgil, B. Enhancement of titanium dioxide photocatalysis by water-soluble fullerenes. J. Colloid Interface Sci. 2006, 304, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Krishna, V.; Yanes, D.; Imaram, W.; Angerhofer, A.; Koopman, B.; Moudgil, B. Mechanism of enhanced photocatalysis with polyhydroxy fullerenes. Appl. Catal. B 2008, 79, 376–381. [Google Scholar] [CrossRef]
- Park, Y.; Singh, N.J.; Kim, K.S.; Tachikawa, T.; Majima, T.; Choi, W. Fullerol-Titania Charge-Transfer-Mediated Photocatalysis Working under Visible Light. Chem. A Eur. J. 2009, 15, 10843–10850. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Lee, C.; Lee, K.H.; Yoon, K.B. 1:1 and 2:1 charge-transfer complexes between aromatic hydrocarbons and dry titanium dioxide. Angew. Chem. Int. Ed. Engl. 2005, 44, 910–913. [Google Scholar] [CrossRef]
- Ramakrishnan, A.; Neubert, S.; Mei, B.; Strunk, J.; Wang, L.; Bledowski, M.; Muhler, M.; Beranek, R. Enhanced performance of surface-modified TiO2 photocatalysts prepared via a visible-light photosynthetic route. Chem. Commun. 2012, 48, 8556–8558. [Google Scholar] [CrossRef]
- Velmurugan, R.; Swaminathan, M. An efficient nanostructured ZnO for dye sensitized degradation of Reactive Red 120 dye under solar light. Sol. Energy Mater. Sol. Cells 2011, 95, 942–950. [Google Scholar] [CrossRef]
- Chen, D.; Ye, J. Hierarchical WO3 Hollow Shells: Dendrite, Sphere, Dumbbell, and Their Photocatalytic Properties. Adv. Funct. Mater. 2008, 18, 1922–1928. [Google Scholar] [CrossRef]
- Xie, X.; Yang, H.; Zhang, F.; Li, L.; Ma, J.; Jiao, H.; Zhang, J. Synthesis of hollow microspheres constructed with α-Fe2O3 nanorods and their photocatalytic and magnetic properties. J. Alloys Compd. 2009, 477, 90–99. [Google Scholar] [CrossRef]
- Bae, K.W. Synthesis of SnO2-Mn-C60 Nanocomposites and Their Photocatalytic Activity for Degradation of Organic Dyes. Elastom. Compos. 2017, 52, 287–294. [Google Scholar]
- Di Paola, A.; Garcia-Lopez, E.; Marci, G.; Palmisano, L. A survey of photocatalytic materials for environmental remediation. J. Hazard. Mater. 2012, 211, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.K.; Lee, J.H.; Cho, B.H.; Ko, W.B. Preparation of a 70 fullerene-ZnO nanocomposite in an electric furnace and photocatalytic degradation of organic dyes. J. Ceram. Process. Res. 2011, 12, 212–217. [Google Scholar]
- Chang, K.; Mei, Z.; Wang, T.; Kang, Q.; Ouyang, S.; Ye, J. MoS2/Graphene Cocatalyst for Efficient Photocatalytic H2 Evolution under Visible Light Irradiation. ACS Nano 2014, 8, 7078–7087. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, H.; Wang, J.; Zeng, G.; Chen, X.; Wu, Z.; Jiang, L.; Xiong, T.; Zhang, J.; Wang, H. Near-infrared-driven Cr(vi) reduction in aqueous solution based on a MoS2/Sb2S3 photocatalyst. Catal. Sci. Technol. 2018, 8, 1545–1554. [Google Scholar] [CrossRef]
- Yuan, X.; Jiang, L.; Liang, J.; Pan, Y.; Zhang, J.; Wang, H.; Leng, L.; Wu, Z.; Guan, R.; Zeng, G. In-situ synthesis of 3D microsphere-like In2S3/InVO4 heterojunction with efficient photocatalytic activity for tetracycline degradation under visible light irradiation. Chem. Eng. J. 2019, 356, 371–381. [Google Scholar] [CrossRef]
- Simon, T.; Bouchonville, N.; Berr, M.J.; Vaneski, A.; Adrović, A.; Volbers, D.; Wyrwich, R.; Döblinger, M.; Susha, A.S.; Rogach, A.L.; et al. Redox shuttle mechanism enhances photocatalytic H2 generation on Ni-decorated CdS nanorods. Nat. Mater. 2014, 13, 1013–1018. [Google Scholar] [CrossRef]
- Meng, Z.-D.; Ghosh, T.; Zhu, L.; Choi, J.-G.; Park, C.-Y.; Oh, W.-C. Synthesis of fullerene modified with Ag2S with high photocatalytic activity under visible light. J. Mater. Chem. 2012, 22, 16127–16135. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ji, M.; Wang, B.; Yin, S.; Zhang, Q.; Chen, Z.; Li, H. Advanced photocatalytic performance of graphene-like BN modified BiOBr flower-like materials for the removal of pollutants and mechanism insight. Appl. Catal. B 2016, 183, 254–262. [Google Scholar] [CrossRef]
- Yu, H.; Huang, B.; Wang, H.; Yuan, X.; Jiang, L.; Wu, Z.; Zhang, J.; Zeng, G. Facile construction of novel direct solid-state Z-scheme AgI/BiOBr photocatalysts for highly effective removal of ciprofloxacin under visible light exposure: Mineralization efficiency and mechanisms. J. Colloid Interface Sci. 2018, 522, 82–94. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Wu, Z.; Yu, H.; Mo, D.; Wang, H.; Xiao, Z.; Zhou, C. Nitrogen self-doped g-C3N4 nanosheets with tunable band structures for enhanced photocatalytic tetracycline degradation. J. Colloid Interface Sci. 2019, 536, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Wu, Z.; Wang, H.; Zhang, J.; Xiong, T.; Li, H. A facile band alignment of polymeric carbon nitride isotype heterojunctions for enhanced photocatalytic tetracycline degradation. Environ. Sci. Nano 2018, 5, 2604–2617. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Tu, W.; Wu, S.; Liu, Y.; Tan, Y.Z.; Luo, H.; Yuan, X.; Chew, J.W. Petal-like CdS nanostructures coated with exfoliated sulfur-doped carbon nitride via chemically activated chain termination for enhanced visible-light–driven photocatalytic water purification and H2 generation. Appl. Catal. B 2018, 229, 181–191. [Google Scholar] [CrossRef]
- Ouyang, K.; Dai, K.; Chen, H.; Huang, Q.; Gao, C.; Cai, P. Metal-free inactivation of E. coli O157:H7 by fullerene/C3N4 hybrid under visible light irradiation. Ecotoxicol. Environ. Saf. 2017, 136, 40–45. [Google Scholar] [CrossRef]
- Zhang, S.; Arunachalam, P.; Abe, T.; Iyoda, T.; Nagai, K. Photocatalytic decomposition of N-methyl-2-pyrrolidone, aldehydes, and thiol by biphase and p/n junction-like organic semiconductor composite nanoparticles responsive to nearly full spectrum of visible light. J. Photochem. Photobiol. A Chem. 2012, 244, 18–23. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Tse, D.S.; Malhotra, R.; Lorents, D.C. Solubility of fullerene (C60) in a variety of solvents. J. Phys. Chem. 1993, 97, 3379–3383. [Google Scholar] [CrossRef]
- Kyriakopoulos, J.; Papastavrou, A.T.; Panagiotou, G.D.; Tzirakis, M.D.; Triantafyllidis, K.S.; Alberti, M.N.; Bourikas, K.; Kordulis, C.; Orfanopoulos, M.; Lycourghiotis, A. Deposition of fullerene C-60 on the surface of MCM-41 via the one-step wet impregnation method: Active catalysts for the singlet oxygen mediated photooxidation of alkenes. J. Mol. Catal. A Chem. 2014, 381, 9–15. [Google Scholar] [CrossRef]
- Hou, W.-C.; Jafvert, C.T. Photochemistry of Aqueous C60 Clusters: Evidence of 1O2 Formation and its Role in Mediating C60 Phototransformation. Environ. Sci. Technol. 2009, 43, 5257–5262. [Google Scholar] [CrossRef] [PubMed]
- Tzirakis, M.D.; Vakros, J.; Loukatzikou, L.; Amargianitakis, V.; Orfanopoulos, M.; Kordulis, C.; Lycourghiotis, A. Gamma-Alumina-supported 60 fullerene catalysts: Synthesis, properties and applications in the photooxidation of alkenes. J. Mol. Catal. A Chem. 2010, 316, 65–74. [Google Scholar] [CrossRef]
- Lee, J.; Yamakoshi, Y.; Hughes, J.B.; Kim, J.-H. Mechanism of C60 Photoreactivity in Water: Fate of Triplet State and Radical Anion and Production of Reactive Oxygen Species. Environ. Sci. Technol. 2008, 42, 3459–3464. [Google Scholar] [CrossRef]
- Hotze, E.M.; Labille, J.; Alvarez, P.; Wiesner, M.R. Mechanisms of Photochemistry and Reactive Oxygen Production by Fullerene Suspensions in Water. Environ. Sci. Technol. 2008, 42, 4175–4180. [Google Scholar] [CrossRef] [PubMed]
- Vileno, B.; Sienkiewicz, A.; Lekka, M.; Kulik, A.J.; Forró, L. In vitro assay of singlet oxygen generation in the presence of water-soluble derivatives of C60. Carbon 2004, 42, 1195–1198. [Google Scholar] [CrossRef]
- Cho, M.; Lee, J.; Mackeyev, Y.; Wilson, L.J.; Alvarez, P.J.J.; Hughes, J.B.; Kim, J.-H. Visible Light Sensitized Inactivation of MS-2 Bacteriophage by a Cationic Amine-Functionalized C60 Derivative. Environ. Sci. Technol. 2010, 44, 6685–6691. [Google Scholar] [CrossRef] [PubMed]
- Snow, S.D.; Park, K.; Kim, J.-H. Cationic Fullerene Aggregates with Unprecedented Virus Photoinactivation Efficiencies in Water. Environ. Sci. Technol. Lett. 2014, 1, 290–294. [Google Scholar] [CrossRef]
- Mroz, P.; Tegos, G.P.; Gali, H.; Wharton, T.; Sarna, T.; Hamblin, M.R. Photodynamic therapy with fullerenes. Photochem. Photobiol. Sci. 2007, 6, 1139–1149. [Google Scholar] [CrossRef] [Green Version]
- Hou, W.-C.; Kong, L.; Wepasnick, K.A.; Zepp, R.G.; Fairbrother, D.H.; Jafvert, C.T. Photochemistry of Aqueous C60 Clusters: Wavelength Dependency and Product Characterization. Environ. Sci. Technol. 2010, 44, 8121–8127. [Google Scholar] [CrossRef]
- Hou, W.-C.; Jafvert, C.T. Photochemical Transformation of Aqueous C60 Clusters in Sunlight. Environ. Sci. Technol. 2009, 43, 362–367. [Google Scholar] [CrossRef]
- Lee, J.; Mackeyev, Y.; Cho, M.; Wilson, L.J.; Kim, J.-H.; Alvarez, P.J.J. C60 Aminofullerene Immobilized on Silica as a Visible-Light-Activated Photocatalyst. Environ. Sci. Technol. 2010, 44, 9488–9495. [Google Scholar] [CrossRef]
- Redmond, R.W.; Kochevar, I.E. Symposium-in-Print: Singlet Oxygen Invited Review. Photochem. Photobiol. 2006, 82, 1178–1186. [Google Scholar] [CrossRef]
- Foote, C.S.; Clennan, E.L. Properties and Reactions of Singlet Dioxygen. In Active Oxygen in Chemistry; Foote, C.S., Valentine, J.S., Greenberg, A., Liebman, J.F., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 105–140. [Google Scholar]
- Lee, J.; Hong, S.; Mackeyev, Y.; Lee, C.; Chung, E.; Wilson, L.J.; Kim, J.H.; Alvarez, P.J. Photosensitized oxidation of emerging organic pollutants by tetrakis C(6)(0) aminofullerene-derivatized silica under visible light irradiation. Environ. Sci. Technol. 2011, 45, 10598–10604. [Google Scholar] [CrossRef]
- Choi, Y.; Ye, Y.; Mackeyev, Y.; Cho, M.; Lee, S.; Wilson, L.J.; Lee, J.; Alvarez, P.J.J.; Choi, W.; Lee, J. C60 aminofullerene-magnetite nanocomposite designed for efficient visible light photocatalysis and magnetic recovery. Carbon 2014, 69, 92–100. [Google Scholar] [CrossRef]
- Moor, K.J.; Kim, J.H. Simple synthetic method toward solid supported c60 visible light-activated photocatalysts. Environ. Sci. Technol. 2014, 48, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Rogozea, E.A.; Meghea, A.; Olteanu, N.L.; Bors, A.; Mihaly, M. Fullerene-modified silica materials designed for highly efficient dyes photodegradation. Mater. Lett. 2015, 151, 119–121. [Google Scholar] [CrossRef]
- Wakimoto, R.; Kitamura, T.; Ito, F.; Usami, H.; Moriwaki, H. Decomposition of methyl orange using C60 fullerene adsorbed on silica gel as a photocatalyst via visible-light induced electron transfer. Appl. Catal. B 2015, 166–167, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Kyriakopoulos, J.; Kordouli, E.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Decolorization of Orange-G Aqueous Solutions over C-60/MCM-41 Photocatalysts. Appl. Sci. 2019, 9, 1958. [Google Scholar] [CrossRef] [Green Version]











| Photocatalyst (Additive Amount) | Synthesis Method (Fullerene Content) | Pollutants | Experimental Conditions (Light Source, Pollutant Concentration and React Time) | Photocatalytic Activity | Enhancement Factor | Reference |
|---|---|---|---|---|---|---|
| TiO2/C60 (1 g/L) | In-situ growth (2.0 wt %) | Methylene blue (MB) | UV irradiation, 1.0 × 10−4 mol/L, 60 min | 99% | around 75% for TiO2 | [67] |
| TiO2/C60 (1 g/L) | Ultrasonication–evaporation (1.0 wt %) | RhB | 500 W Xe-lamp (>400 nm), 10 mg/L, 150 min | 95% | below 5% for TiO2 | [68] |
| TiO2/C70 (1 g/L) | Hydrothermal synthesis (8.5 wt %) | Sulfathiazole | 300 W Xenon lamp (>420 nm), 10 mg/mL, 180 min | 80% | 10% for TiO2 | [69] |
| ZnO/C60 (0.5 g/L) | Simple adsorption (1.5 wt %) | MB | 8 W UV lamp (λ = 254 nm), 8 mg/L | k = 0.0569 min−1 | 3-times than ZnO | [70] |
| ZnO/C60 (0.83 g/L) | Chemical vapor (16.7 wt %) | Phenol | 1500 W xenon lamp simulating solar light, 20 mg/L | k = 0.160 min−1 | 1.22-times than ZnO | [42] |
| ZnFe2O4@C60 (1 g/L) | Hydrothermal synthesis | Norfloxacin | Solar irradiation, 20 mL of 50 ppm norfloxacin, 90 min | 85% | 60% for ZnFe2O4 | [71] |
| WO3@C60 | Hydrothermal synthesis (4.0 wt %) | MB | Visible light, 90 min | 94% | Inferior degradation efficiency for pure WO3 | [53] |
| ZnAlTi-LDH@C60 (ZnAlTi-LDO) 0.5 g/L | Precipitation (5%) | Bisphenol A (BPA) | 300 W xenon lamp simulating visible light, 10 mg/L, 60 min | 80% | below 10% for ZnAlTi-LDH | [38] |
| CdS/C60 (1 g/L) | One-pot hydrothermal method (0.4 wt %) | RhB | 300 W xenon lamp (>420 nm), 20 mL, 10 ppm of RhB | k = 0.089 min−1 | 1.5-times than CdS | [43] |
| C3N4/C60 (0.6 g/L) | Simple adsorption (1.0 wt %) | RhB | 500 W xenon lamp (>420 nm), 50 mL, 1.0 × 10−5 mol l−1 RhB, 60 min | 97% | 54% for C3N4 | [45] |
| g-C3N4/C60 (0.5 g/L) | Calcination (0.03 wt %) | MB, phenol | 500 W xenon lamp (>420 nm), MB (50 mL, 0.01 mM), phenol (50 mL, 5 ppm). | k1 = 1.036 h−1, k2 = 0.093 h−1 | 3.2- and 2.9-times than C3N4 | [35] |
| Ag3PO4/C60 (0.5 g/L) | Precipitation (2.0 wt %) | Acid red 18 (AR18) | 400 W halogen lamp (420–780 nm, 21.5–23.0 mW cm−2), 50 mL, 6.5 × 10−5 mol/L of AR18, 60 min | 90% | 53% for Ag3PO4 | [31] |
| Ag3PO4/C60 (1 g/L) | Precipitation (5.0 mg/L) | Methyl Orange (MO) | 300 W xenon lamp (>420 nm), 10 mg/L | k = 0.453 min−1 | k = 0.028 min−1 for Ag3PO4 | [72] |
| PbMoO4/C60 (0.4 g/L) | Hydrothermal synthesis (0.5 wt %) | RhB | 18 W low-pressure mercury lamp as the UV light source, 50 mL of RhB (1 × 10−5 M), 2 h | 99% | 37% for PbMoO4 | [50] |
| Bi2WO6/C60 (1 g/L) | Simple adsorption (1.25 wt %) | MB, RhB | 500 W xenon lamp (>420 nm), 1 × 10−5 mol/L RhB or MB (100 mL) | k1 = 0.0099 min−1, k2 = 0.0454 min−1 | 5.0- and 1.5-times than Bi2WO6 | [40] |
| BiOCl/C70 (1 g/L) | In-situ growth (1.0 wt %) | RhB | 500 W xenon lamp (>420 nm), 10 mg/L, 30 min | 99.8% | 49.7% and 66.4% for BiOCl and P25 (TiO2) | [39] |
| Bi2TiO4F2/C60 | Solvothermal method (1.0 wt %) | RhB | Visible light, 20 ppm RhB, 120 min | 93% | 65% for Bi2TiO4F2 | [73] |
| CNTs/BiVO4-C60 (2 g/L) | Hydrothermal synthesis (2.5 wt %) | RhB | 300 W xenon lamp (>420nm), 100 mL, 0.01 mmol/L RhB, 30 min | 96.1% | 74.0% for BiVO4 | [51] |
| CNTs/Bi2MoO6-C60 (2 g/L) | Hydrothermal synthesis (2.5 wt %) | RhB | 300 W xenon lamp (>420 nm), 100 mL, 0.01 mmol/L RhB, 30 min | 88.4% | 43.7% for Bi2MoO6 | [51] |
| Ag3PO4/Fe3O4/C60 (1 g/L) | Hydrothermal synthesis (5.0 wt %) | MB | 400 W mercury lamp (>420 nm), 50 mL of MB (25 mg/L), 300 min | 95% | 33% for Ag3PO4 | [74] |
| TiO2/Pt-C60 (1 g/L) | Sol-gel method (7.5 wt %) | MO | 8 W halogen lamp (400–790 nm), 50 mL, 1 × 10−5 mol/L of MO | k = 3.67×10−3 min−1 | 1.58- and 16.4-times than Pt/TiO2 and TiO2 | [75] |
| TiO2/Pd-C60 (1 g/L) | Sol-gel method (21 wt %) | MB | UV lamp box (8 W, 365 nm), 50 mL, 1 × 10−4 mol/L of MB | k = 0.0337 min−1 | 14-times than TiO2 | [76] |
| Au/TiO2-C60 (1 g/L) | Hydrothermal synthesis (3.25 wt %) | MO | 500W tungsten halogen lamp, 20 mL, 10 mg/L of MO, 160 min | 95% | 47% for TiO2 | [77] |
| TiO2/CdS-C60 (1 g/L) | Sol-gel method (5.0 wt %) | MB | 8 W halogen lamp (400–790 nm), 50 mL, 1 × 10−5 mol/L of MB | k = 7.9×10−3 min−1 | 4.9- and 3.5-times than CdS/TiO2 and TiO2 | [56] |
| TiO2/WO3-C60 (1 g/L) | Sol-gel method (3.0 wt %) | MO | 8 W halogen lamp (400–790 nm), 50 mL, 1 × 10−5 mol/L of MO | k = 4.75×10−3 min−1 | 1.66- and 21.2-times than WO3/TiO2 and TiO2 | [78] |
| TiO2/CD/C60 (1 g/L) | Simple adsorption (1.5%) | MB, 4-chlorophenol (4-CP) | 84 W light sources (>420 nm), MB (10 mL, 144 μM), 10 mg/L 4-CP | k1 = 0.014 min−1, k2 = 0.036 min−1 | 2- and 4.9-times than TiO2 | [79] |
| TiO2/Fullerol (1 g/L) | Wet impregnation | Procion red MX-5B | 16 solar UVA lamps (350 nm) | k = 0.0128 min−1 | 2.6-times than TiO2 | [80] |
| TiO2/Fullerol (1 g/L) | Wet impregnation (1.0 wt %) | Formic acid | Hg lamp (365 nm) | k = 91.0 µmol L−1 min−1 | 1.3-times than TiO2 | [59] |
| Nb-TiO2/Fullerol (0.5 g/L) | Simple adsorption | 4-chlorophenol | 300-W Xe arc lamp (>420 nm) | k = 13.9×10−3 min−1 | 3.3-times than P25 | [81] |
| Photocatalyst (Additive Amount) | Synthesis Method (Fullerene Content) | Experimental Conditions | Photocatalytic Rate of H2 Generation | Enhancement Factor | Reference |
|---|---|---|---|---|---|
| CdS/C60 (0.5 g/L) | Hydrothermal synthesis (0.4 wt %) | 300 W xenon lamp (>420 nm), 50 mL aqueous solution containing 10 vol% lactic acid and 1 wt % Pt | 1.73 mmol h−1 g−1 | 2.3 Times of pure CdS | [43] |
| WO3@C60 (0.5 g/L) | Hydrothermal synthesis (4 wt %) | 300 W xenon lamp (>420 nm), Triethanolamine (TEA) | 154 µmol h−1 g−1 | 2 times of pure WO3 | [53] |
| MoS2/C60 (0.5 g/L) | Ball milling method (2.8 wt %) | 300 W xenon lamp (>420 nm), 20 mL aqueous solution containing 3.5 mg Eosin Y (EY) and 1 mL TEA | 6.89 mmol h−1 g−1 | 9.3 times of ball-milled MoS2 | [54] |
| g-C3N4/C60 (1 g/L) | Ball milling method (12 wt %) | 300 W xenon lamp (>420 nm), 100 mL aqueous solution containing 17.5 mg EY and 5 mL TEA | 266 µmol h−1 g−1 | 4.0 times higher than pristine C3N4 | [55] |
| Cr1.3Fe0.7O3-C60 (5 mg/78 mL) | Simple adsorption (3%) | 300 W xenon lamp (>420 nm), 78 mL 10 vol% TEA aqueous solution | 220.5 µmol h−1 g−1 | 2 times of the Cr1.3Fe0.7O3 | [82] |
| Fe2O3/C60 (5 mg/78 mL) | Simple adsorption (0.5~1 wt %) | 300 W xenon lamp (>420 nm), 78 mL 10 vol% TEA aqueous solution | β-Fe2O3/C60: 1665 µmol h−1 g−1; α-Fe2O3/C60: 202.9 µmol h−1 g−1; γ-Fe2O3/C60: 169.4 µmol h−1 g−1 | β-Fe2O3: 169.4 µmol h−1 g−1; α-Fe2O3: 80.6 µmol h−1 g−1; γ-Fe2O3: 252 µmol h−1 g−1; C3N4: 82.7 µmol h−1 g−1 | [83] |
| CdS/TiO2-C60 (50 mg/80 mL) | An ion-exchanged method (0.5 wt %) | Low power UV-LEDs (420 nm), 80 mL solution (0.25 M Na2S, 0.25 M Na2SO3) | 120.6 µmol h−1 g−1 | 8.5 times of CdS/TiO2 | [84] |
| TiO2/C60-d-CNTs (1 g/L) | Hydrothermal synthesis (5 wt %) | 300 W xenon lamp (>420 nm), 100 mL 10 vol% TEA aqueous solution | 651 µmol h−1 g−1 | 208 µmol h−1 g−1 for pure TiO2 | [85] |
| g-C3N4/graphene/ C60 (2 g/L) | Wet impregnation | Light-emitting diode (>420 nm), 50 mL solution containing 1 wt‰ Pt and 10 vol% TEA | 545 µmol h−1 g−1 | 50.8 and 4.24 times of graphene/g-C3N4 and C60/g-C3N4 | [19] |
| (2TPABTz)–metal complex/C60 | Simple adsorption (2 wt %) | 300 W xenon lamp (>420 nm), an aqueous lactic acid (LA) | 2TPABTz-Cu/C60: 4.05 mmol h−1 g−1; 2TPABTz-Co/C60: 3.77 mmol h−1 g−1; 2TPABTz-Ru/C60: 6.12 mmol h−1 g−1 | 2TPABTz-Cu: 4.05 mmol h−1 g−1; 2TPABTz-Co: 3.77 mmol h−1 g−1; 2TPABTz-Ru: 6.12 mmol h−1 g−1; TiO2 (P25): 0.072 mmol h−1 g−1 | [86] |
| WO3/C60@Ni3B/Ni(OH)2 2 g/L | Photo-deposition technique | 500 W xenon lamp (>420nm), 100 mL 10 vol% TEA aqueous solution | 1.578 mmol h−1 g−1 | 9.6 times of pure photocatalyst | [87] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, S.; Yuan, X.; Jiang, L.; Xiong, T.; Zhang, J. Recent Progress on Fullerene-Based Materials: Synthesis, Properties, Modifications, and Photocatalytic Applications. Materials 2020, 13, 2924. https://doi.org/10.3390/ma13132924
Yao S, Yuan X, Jiang L, Xiong T, Zhang J. Recent Progress on Fullerene-Based Materials: Synthesis, Properties, Modifications, and Photocatalytic Applications. Materials. 2020; 13(13):2924. https://doi.org/10.3390/ma13132924
Chicago/Turabian StyleYao, Sai, Xingzhong Yuan, Longbo Jiang, Ting Xiong, and Jin Zhang. 2020. "Recent Progress on Fullerene-Based Materials: Synthesis, Properties, Modifications, and Photocatalytic Applications" Materials 13, no. 13: 2924. https://doi.org/10.3390/ma13132924