Tuning the Integration Rate of Ce(Ln)O2 Nanoclusters into Nanoparticulated ZrO2 Supports: When the Cation Size Matters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZrO2-CeO2 and ZrO2-Ce0.87Ln0.13O1.935 Nanostructured Materials
2.2. Thermochemical Ageing Treatments
2.3. Characterization of the Fresh and Thermally Aged ZrO2-CeO2 and ZrO2-Ce0.87Ln0.13O1.935 Samples
2.3.1. Chemical Composition
2.3.2. X-ray Diffraction
2.3.3. Textural Characterization
2.3.4. Surface Chemical Characterization
2.3.5. Electron Microscopy Characterization
2.3.6. Reducibility Measurements
3. Results and Discussion
3.1. Characterization of the Fresh Undoped and Ln-Doped Nanostructured ZrO2-CeO2 Samples
3.2. Characterization of the Thermally Aged Undoped and Ln-Doped ZrO2-CeO2 Samples
3.2.1. Textural Characterization
3.2.2. Structural Characterization
3.2.3. Redox Behaviour
3.2.4. Surface Chemical Characterization
4. Conclusions
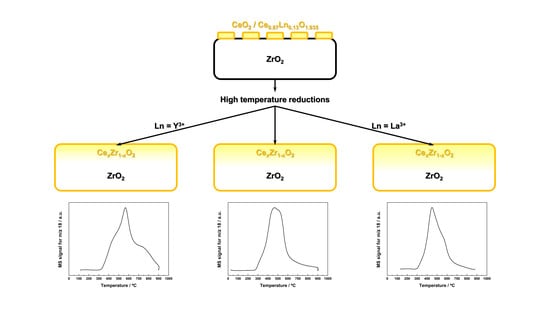
- Irrespective of whether the supported CeO2 phase is doped or not, and of the nature of the Ln3+ dopant, a reduction treatment at a minimum temperature of 900 °C was required to accomplish a significant diffusion of cerium inside the ZrO2 crystallites, with the inherent formation of cerium-zirconium mixed oxide surface phases.
- The size of the dopant was found to noticeably affect the extent of the diffusion process. As compared to the undoped ZrO2-CeO2 nanostructured catalyst, Y3+ incorporation slightly hindered the cerium diffusion, thereby decreasing the maximum depth to which the corresponding mixed oxide phases are formed, while the opposite effect was observed for the La3+-doped nanocatalyst.
- Such differences in cerium diffusion led to changes in both surface and nanostructural features of the oxides, which were tentatively correlated with the redox response of the thermally aged materials. Thus, the enhanced reducibility in the low temperature range exhibited by the La3+-modified nanocatalyst after applying the whole set of consecutive reduction treatments was associated with a greater formation of mixed oxide surface phases. In stark contrast, the slight worsening in reducibility found for the Y3+-containing aged sample was interpreted on the basis of a lower amount of the abovementioned surface phases.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trovarelli, A. Catalysis by Ceria and Related Materials, 1st ed.; Imperial College Press: London, UK, 2002; pp. 1–508. [Google Scholar]
- Trovarelli, A.; Fornasiero, P. Catalysis by Ceria and Related Materials, 2nd ed.; Imperial College Press: London, UK, 2013; pp. 1–888. [Google Scholar]
- Trovarelli, A. Structural and oxygen storage/release properties of CeO2-based solid solutions. Comments Inorg. Chem. A J. Crit. Discuss. Curr. Lit. 1999, 20, 263–284. [Google Scholar] [CrossRef]
- Colón, G.; Valdivieso, F.; Pijolat, M.; Baker, R.T.; Calvino, J.J.; Bernal, S. Textural and phase stability of CexZr1−xO2 mixed oxides under high temperature oxidising conditions. Catal. Today 1999, 50, 271–284. [Google Scholar] [CrossRef]
- Bernal, S.; Blanco, G.; Calvino, J.J.; Hernández, J.C.; Pérez-Omil, J.A.; Pintado, J.M.; Yeste, M.P. Some recent results on the correlation of nano-structural and redox properties in ceria-zirconia mixed oxides. J. Alloys Compd. 2008, 451, 521–525. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Graziani, M. Use of CeO2-based oxides in the three-way catalysis. Catal. Today 1999, 50, 285–298. [Google Scholar] [CrossRef]
- Monte, R.D.; Kašpar, J. Nanostructured CeO2–ZrO2 mixed oxides. J. Mater. Chem. 2005, 15, 633–648. [Google Scholar] [CrossRef]
- Yeste, M.P.; Hernández, J.C.; Bernal, S.; Blanco, G.; Calvino, J.J.; Pérez-Omil, J.A.; Pintado, J.M. Redox behavior of thermally aged ceria-zirconia mixed oxides. Role of their surface and bulk structural properties. Chem. Mater. 2006, 18, 2750–2757. [Google Scholar] [CrossRef]
- Vidmar, P.; Fornasiero, P.; Kašpar, J.; Gubitosa, G.; Graziani, M. Effects of trivalent dopants on the redox properties of Ce0.6Zr0.4O2 mixed oxide. J. Catal. 1997, 171, 160–168. [Google Scholar] [CrossRef]
- Ikryannikova, L.N.; Aksenov, A.A.; Markaryan, G.L.; Murav’eva, G.P.; Kostyuk, B.G.; Kharlanov, A.N.; Lunina, E.V. The red–ox treatments influence on the structure and properties of M2O3–CeO2–ZrO2 (M = Y, La) solid solutions. Appl. Catal. A Gen. 2001, 210, 225–235. [Google Scholar] [CrossRef]
- He, H.; Dai, H.X.; Ng, L.H.; Wong, K.W.; Au, C.T. Pd-, Pt-, and Rh-loaded Ce0.6Zr0.35Y0.05O2 three-way catalysts: An investigation on performance and redox properties. J. Catal. 2002, 206, 1–13. [Google Scholar] [CrossRef]
- He, H.; Dai, H.X.; Wong, K.W.; Au, C.T. RE0.6Zr0.4−xYxO2 (RE = Ce, Pr; X = 0, 0.05) solid solutions: An investigation on defective structure, oxygen mobility, oxygen storage capacity, and redox properties. Appl. Catal. A Gen. 2003, 251, 61–74. [Google Scholar] [CrossRef]
- Ikryannikova, L.N.; Markaryan, G.L.; Kharlanov, A.N.; Lunina, E.V. Electron-accepting surface properties of ceria-(praseodymia)-zirconia solids modified by Y3+ or La3+ studied by paramagnetic probe method. Appl. Surf. Sci. 2003, 207, 100–114. [Google Scholar] [CrossRef]
- Turko, G.A.; Ivanova, A.S.; Plyasova, L.M.; Litvak, G.S.; Rogov, V.A. Synthesis and characterization of fluorite-like Ce–Zr–Y–La–O systems. Kinet. Catal. 2005, 46, 884–890. [Google Scholar] [CrossRef]
- Yucai, H.; Ping, Y.; Tao, L.; Wei, J.; Bing, L. Rare earth doping effects on properties of ceria-zirconia solid solution. J. Rare Earths 2006, 24, 86–89. [Google Scholar] [CrossRef]
- Aneggi, E.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Promotional effect of rare earths and transition metals in the combustion of diesel soot over CeO2 and CeO2-ZrO2. Catal. Today 2006, 114, 40–47. [Google Scholar] [CrossRef]
- Fan, J.; Wu, X.; Yang, L.; Weng, D. The SMSI between supported platinum and CeO2-ZrO2-La2O3 mixed oxides in oxidative atmosphere. Catal. Today 2007, 126, 303–312. [Google Scholar] [CrossRef]
- Si, R.; Zhang, Y.-W.; Wang, L.-M.; Li, S.-J.; Lin, B.-X.; Chu, W.-S.; Wu, Z.-Y.; Yan, C.-H. Enhanced thermal stability and oxygen storage capacity for CexZr1−xO2 (x = 0.4–0.6) solid solutions by hydrothermally homogenous doping of trivalent rare earths. J. Phys. Chem. C 2007, 111, 787–794. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Hu, Y.; Wang, M.; Li, H. Effect of doping elements on catalytic performance of CeO2-ZrO2 solid solutions. J. Rare Earths 2008, 26, 357–361. [Google Scholar] [CrossRef]
- Dong, F.; Tanabe, T.; Suda, A.; Takahashi, N.; Sobukawa, H.; Shinjoh, H. Investigation of the OSC performance of Pt/CeO2-ZrO2-Y2O3 catalysts by CO oxidation and 18O/16O isotopic exchange reaction. Chem. Eng. Sci. 2008, 63, 5020–5027. [Google Scholar] [CrossRef]
- Guo, J.; Shi, Z.; Wu, D.; Yin, H.; Chen, Y. A comparative study of Y3+- or/and La3+-doped CeO2-ZrO2-based solid solution. J. Mater. Res. 2013, 28, 887–896. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Q.; Li, G.; Zhou, R. Effect of rare earth (La, Nd, Pr, Sm and Y) on the performance of Pd/Ce0.67Zr0.33MO2-δ three-way catalysts. J. Env. Chem. Eng. 2013, 1, 534–543. [Google Scholar] [CrossRef]
- Balducci, G.; Fornasiero, P.; Di Monte, R.; Kašpar, J.; Meriani, S.; Graziani, M. An unusual promotion of the redox behaviour of CeO2-ZrO2 solid solutions upon sintering at high temperatures. Catal. Lett. 1995, 33, 193–200. [Google Scholar] [CrossRef]
- Otsuka-Yao-Matsuo, S.; Omata, T.; Izu, N.; Kishimoto, H. Oxygen release behavior of CeZrO4 powders and appearance of new compounds κ and t*. J. Solid State Chem. 1998, 138, 47–54. [Google Scholar] [CrossRef]
- Omata, T.; Kishimoto, H.; Otsuka-Yao-Matsuo, S.; Ohtori, N.; Umesaki, N. Vibrational spectroscopic and X-ray diffraction studies of cerium zirconium oxides with Ce/Zr composition ratio = 1 prepared by reduction and successive oxidation of t′-(Ce0.5Zr0.5)O2 Phase. J. Solid State Chem. 1999, 147, 573–583. [Google Scholar] [CrossRef]
- Baker, R.T.; Bernal, S.; Blanco, G.; Cordón, A.M.; Pintado, J.M.; Rodríguez-Izquierdo, J.M.; Fally, F.; Perrichon, V. Reversible changes in the redox behaviour of a Ce0.68Zr0.32O2 mixed oxide: Effect of alternating the re-oxidation temperature after reduction at 1223 K. Chem. Commun. 1999, 149–150. [Google Scholar] [CrossRef]
- Fornasiero, P.; Montini, T.; Graziani, M.; Kašpar, J.; Hungría, A.B.; Martínez-Arias, A.; Conesa, J.C. Effects of thermal pretreatment on the redox behaviour of Ce0.5Zr0.5O2: Isotopic and spectroscopic studies. Phys. Chem. Chem. Phys. 2002, 4, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Montini, T.; Hickey, N.; Fornasiero, P.; Graziani, M.; Bañares, M.A.; Martinez-Huerta, M.V.; Alessandri, I.; Depero, L.E. Variations in the extent of pyrochlore-type cation ordering in Ce2Zr2O8: A t′-κ pathway to low-temperature reduction. Chem. Mater. 2005, 17, 1157–1166. [Google Scholar] [CrossRef]
- Zhao, M.; Shen, M.; Wang, J. Effect of surface area and bulk structure on oxygen storage capacity of Ce0.67Zr0.33O2. J. Catal. 2007, 248, 258–267. [Google Scholar] [CrossRef]
- Yeste, M.P.; Primus, P.A.; Alcantara, R.; Cauqui, M.A.; Calvino, J.J.; Pintado, J.M.; Blanco, G. Surface characterization of two Ce0.62Zr0.38O2 mixed oxides with different reducibility. Appl. Surf. Sci. 2020, 503, 144255. [Google Scholar] [CrossRef]
- Izu, N.; Omata, T.; Otsuka-Yao-Matsuo, S. Oxygen release behaviour of Ce(1−x)ZrxO2 powders and appearance of Ce(8−4y)Zr4yO(14−δ) solid solution in the ZrO2-CeO2-CeO1.5 system. J. Alloys Compd. 1998, 270, 107–114. [Google Scholar] [CrossRef]
- Sasaki, T.; Ukyo, Y.; Kuroda, K.; Arai, S.; Saka, H. Microstructural investigation of ceria-zirconia solid solution with oxygen vacancies. J. Electron. Microsc. 2003, 52, 309–312. [Google Scholar] [CrossRef]
- Montini, T.; Bañares, M.A.; Hickey, N.; Di Monte, R.; Fornasiero, P.; Kašpar, J.; Graziani, M. Promotion of reduction in Ce0.5Zr0.5O2: The pyrochlore structure as effect rather than cause? Phys. Chem. Chem. Phys. 2004, 6, 1–3. [Google Scholar] [CrossRef]
- Pérez-Omil, J.A.; Bernal, S.; Calvino, J.J.; Hernández, J.C.; Mira, C.; Rodríguez-Luque, M.P.; Erni, R.; Browning, N.D. Combined HREM and HAADF scanning transmission electron microscopy: A powerful tool for investigating structural changes in thermally aged ceria−zirconia mixed oxides. Chem. Mater. 2005, 17, 4282–4285. [Google Scholar] [CrossRef]
- Kishimoto, H.; Omata, T.; Otsuka-Yao-Matsuo, S.; Ueda, K.; Hosono, H.; Kawazoe, H. Crystal structure of metastable κ-CeZrO4 phase possessing an ordered arrangement of Ce and Zr ions. J. Alloys Compd. 2000, 312, 94–103. [Google Scholar] [CrossRef]
- Yeste, M.P.; Hernández, J.C.; Trasobares, S.; Bernal, S.; Blanco, G.; Calvino, J.J.; Pérez-Omil, J.A.; Pintado, J.M. First stage of thermal aging under oxidizing conditions of a Ce0.62Zr0.38O2 mixed oxide with an ordered cationic sublattice: A chemical, nanostructural, and nanoanalytical study. Chem. Mater. 2008, 20, 5107–5113. [Google Scholar] [CrossRef]
- Yashima, M.; Arashi, H.; Kakihana, M.; Yoshimura, M. Raman scattering study of cubic-tetragonal phase transition in Zr1-xCexO2 solid solution. J. Am. Ceram. Soc. 1994, 77, 1067–1071. [Google Scholar] [CrossRef]
- Yeste, M.P.; Hernández-Garrido, J.C.; Arias, D.C.; Blanco, G.; Rodríguez-Izquierdo, J.M.; Pintado, J.M.; Bernal, S.; Pérez-Omil, J.A.; Calvino, J.J. Rational design of nanostructured, noble metal free, ceria-zirconia catalysts with outstanding low temperature oxygen storage capacity. J. Mater. Chem. A 2013, 1, 4836–4844. [Google Scholar] [CrossRef]
- Arias-Duque, C.; Bladt, E.; Muñoz, M.A.; Hernández-Garrido, J.C.; Cauqui, M.A.; Rodríguez-Izquierdo, J.M.; Blanco, G.; Bals, S.; Calvino, J.J.; Pérez-Omil, J.A.; et al. Improving the redox response stability of ceria-zirconia nanocatalysts under harsh temperature conditions. Chem. Mater. 2017, 29, 9340–9350. [Google Scholar] [CrossRef]
- Wall, F. Rare Earth Elements. In Critical Metals Handbook; Gun, G., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 312–339. ISBN 978-0-470-67171-9. [Google Scholar]
- Goodenough, K.M.; Wall, F.; Merriman, D. The rare earth elements: Demand, global resources, and challenges for resourcing future generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Barroso-Bogeat, A.; Núñez-Pérez, B.; Blanco, G.; Pintado, J.M.; Hernández-Garrido, J.C.; Calvino, J.J. Surface and redox characterization of new nanostructured ZrO2@CeO2 systems with potential catalytic applications. Surf. Interface Anal. 2018, 50, 1025–1029. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Cryst. 1969, B25, 925–946. [Google Scholar] [CrossRef]
- Bernal, S.; Blanco, G.; Cauqui, M.A.; Cifredo, G.A.; Pintado, J.M.; Rodriguez-Izquierdo, J.M. Influence of reduction treatment on the structural and redox behaviour of ceria, La/Ce and Y/Ce mixed oxides. Catal. Lett. 1998, 53, 51–57. [Google Scholar] [CrossRef]
- Artini, C. Rare-earth-doped ceria systems and their performance as solid electrolytes: A puzzling tangle of structural issues at the average and local scale. Inorg. Chem. 2018, 57, 13047–13062. [Google Scholar] [CrossRef] [Green Version]
- Coduri, M.; Checchia, S.; Longhi, M.; Ceresoli, D.; Scavini, M. Rare earth doped ceria: The complex connection between structure and properties. Front. Chem. 2018, 6, 526. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Long, R.; Chen, Y.; Chen, Z. Synthesis, characterization of CeO2@SiO2 nanoparticles and their oxide CMP behavior. Microelectron. Eng. 2010, 87, 1716–1720. [Google Scholar] [CrossRef]
- Cabeza, I.; Souto, L.G.; Pintado, J.M.; Pereira, C.; Freire, C.; Blanco, G. Influence of ceria distribution on the redox behaviour of nanoparticulated CeO2-SiO2 systems with application in catalysis. Surf. Interface Anal. 2014, 46, 712–715. [Google Scholar] [CrossRef]
- Blanco, G.; Pintado, J.M.; Aboussaïd, K.; Cifredo, G.A.; el Begrani, M.S.; Bernal, S. Effect of different alumina dopants on the redox deactivation produced by structural modifications on CePrOx/Al2O3 systems. Catal. Today 2012, 180, 184–189. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Hashimoto, S.; Hirokawa, K.; Fukuda, Y.; Suzuki, K.; Suzuki, T.; Usuki, N.; Gennai, N.; Yoshida, S.; Koda, M.; Sezaki, H.; et al. Correction of peak shift and classification of change of X-ray photoelectron spectra of oxides as a result of ion sputtering. Surf. Interface Anal. 1992, 18, 799–806. [Google Scholar] [CrossRef]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. V. data for 14 organic compounds over the 50-2000 eV range. Surf. Interface Anal. 1993, 21, 165–176. [Google Scholar] [CrossRef]
- Hertl, W. Surface chemistry of zirconia polymorphs. Langmuir 1989, 5, 96–100. [Google Scholar] [CrossRef]
- Duran, P.; Gonzalez, M.; Moure, C.; Jurado, J.R.; Pascual, C. A new tentative phase equilibrium diagram for the ZrO2-CeO2 system in air. J. Mater. Sci. 1990, 25, 5001–5006. [Google Scholar] [CrossRef]
- Adamski, A.; Jakubus, P.; Sojka, Z. Structural and textural evolution of zirconia nanocrystals induced by thermal treatment. Mater. Sci. 2008, 26, 373–380. [Google Scholar]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, 2nd ed.; Wiley: New York, NY, USA, 1974; ISBN 978-0-471-49369-3. [Google Scholar]
- Wallenberg, R.; Withers, R.; Bevan, D.J.M.; Thompson, J.G.; Barlow, P.; Hyde, B.G. The fluorite-related “solid solutions” of CeO2-Y2O3 I: A re-examination by electron microscopy and diffraction. J. Less Common Met. 1989, 156, 1–16. [Google Scholar] [CrossRef]
- Morris, B.C.; Flavell, W.R.; Mackrodtb, W.C.; Morris, M.A. Lattice parameter changes in the mixed-oxide system Ce1−xLaxO2−x/2: A combined experimental and theoretical study. J. Mater. Chem. 1993, 3, 1007–1013. [Google Scholar] [CrossRef]
- Blanco, G. Alternativas Al CeO2 Como Componente De Catalizadores De Tres Vías: Óxidos Mixtos De La/Ce, Y/Ce y Tb/Ce. Ph.D. Thesis, University of Cadiz, Cádiz, Spain, 1997. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.C.; Yao, Y.F.Y. Ceria in automotive exhaust catalysts. I. oxygen storage. J. Catal. 1984, 86, 254–265. [Google Scholar] [CrossRef]
- Giordano, F.; Trovarelli, A.; de Leitenburg, C.; Giona, M. A Model for the temperature-programmed reduction of low and high surface area ceria. J. Catal. 2000, 193, 273–282. [Google Scholar] [CrossRef]
- Xu, J.; Harmer, J.; Li, G.; Chapman, T.; Collier, P.; Longworth, S.; Tsang, S.C. Size dependent oxygen buffering capacity of ceria nanocrystals. Chem. Commun. 2010, 46, 1887–1889. [Google Scholar] [CrossRef]
- Désaunay, T.; Bonura, G.; Chiodo, V.; Freni, S.; Couzinié, J.-P.; Bourgon, J.; Ringuedé, A.; Labat, F.; Adamo, C.; Cassir, M. Surface-dependent oxidation of H2 on CeO2 surfaces. J. Catal. 2013, 297, 193–201. [Google Scholar] [CrossRef]
- Fernandez-Garcia, S.; Jiang, L.; Tinoco, M.; Hungria, A.B.; Han, J.; Blanco, G.; Calvino, J.J.; Chen, X. Enhanced hydroxyl radical scavenging activity by doping lanthanum in ceria nanocubes. J. Phys. Chem. C 2016, 120, 1891–1901. [Google Scholar] [CrossRef]
- Tinoco, M.; Fernandez-Garcia, S.; Villa, A.; Gonzalez, J.M.; Blanco, G.; Hungria, A.B.; Jiang, L.; Prati, L.; Calvino, J.J.; Chen, X. Selective oxidation of glycerol on morphology controlled ceria nanomaterials. Catal. Sci. Technol. 2019, 9, 2328–2334. [Google Scholar] [CrossRef]
- Trovarelli, A.; de Leitenburg, C.; Dolcetti, G.; Llorca, J. CO2 Methanation under transient and steady-state conditions over Rh/CeO2 and CeO2-promoted Rh/SiO2: The role of surface and bulk ceria. J. Catal. 1995, 151, 111–124. [Google Scholar] [CrossRef]
- Bernal, S.; Blanco, G.; Calvino, J.J.; Pérez Omil, J.A.; Pintado, J.M. Some major aspects of the chemical behavior of rare earth oxides: An overview. J. Alloys Compd. 2006, 408–412, 496–502. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Kobune, M.; Gotoh, H. Basic properties of rare earth oxides. Appl. Catal. A Gen. 2009, 356, 57–63. [Google Scholar] [CrossRef]
- Bernal, S.; Blanco, G.; Cifredo, G.; Pérez-Omil, J.A.; Pintado, J.M.; Rodríguez-Izquierdo, J.M. Reducibility of ceria-lanthana mixed oxides under temperature programmed hydrogen and inert gas flow conditions. J. Alloys Compd. 1997, 250, 449–454. [Google Scholar] [CrossRef]
- Vidal, H.; Kašpar, J.; Pijolat, M.; Colon, G.; Bernal, S.; Cordón, A.; Perrichon, V.; Fally, F. Redox behavior of CeO2–ZrO2 mixed oxides I. Influence of redox treatments on high surface area catalysts. Appl. Catal. B Env. 2000, 27, 49–63. [Google Scholar] [CrossRef]
- Vidal, H.; Kašpar, J.; Pijolat, M.; Colon, G.; Bernal, S.; Cordón, A.; Perrichon, V.; Fally, F. Redox behaviour of CeO2-ZrO2 mixed oxides II. Influence of redox treatments on low surface area catalysts. Appl. Catal. B Env. 2001, 30, 75–85. [Google Scholar] [CrossRef]
- Ou, D.R.; Mori, T.; Ye, F.; Zou, J.; Auchterlonie, G.; Drennan, J. Evidence of intragranular segregation of dopant cations in heavily yttrium-doped ceria. Electrochem. Solid State Lett. 2007, 10, P1–P3. [Google Scholar] [CrossRef]
- Yan, P.F.; Mori, T.; Suzuki, A.; Wu, Y.Y.; Auchterlonie, G.J.; Zou, J.; Drennan, J. Grain boundary’s conductivity in heavily yttrium doped ceria. Solid State Ion. 2012, 222–223, 31–37. [Google Scholar] [CrossRef]
- Tabakova, T.; Ilieva, L.; Ivanov, I.; Manzoli, M.; Zanella, R.; Petrova, P.; Kaszkur, Z. Structure-activity relationship in water-gas shift reaction over gold catalysts supported on Y-doped ceria. J. Rare Earths 2019, 37, 383–392. [Google Scholar] [CrossRef]











| Sample | ZrO2/mol% | CeO2/mol% | Ln2O3/mol% | Ce0.87Ln0.13O1.935/mol% | Ln/Ce/at.% |
|---|---|---|---|---|---|
| ZrO2-CeO2 | 83.4 | 16.6 | - | - | - |
| ZrO2-Ce0.87Y0.13O1.935 | 85.8 | 12.9 | 1.3 | 14.2 | 14.2 |
| ZrO2-Ce0.87La0.13O1.935 | 86.5 | 11.8 | 1.8 | 13.5 | 14.6 |
| Sample | (Ce 4d + Ln Xd)/Zr 3d | (Ce + Ln)/Zr 1/at.% |
|---|---|---|
| ZrO2-CeO2 | 0.99 | 0.20 |
| ZrO2-Ce0.87Y0.13O1.935 | 1.29 | 0.18 |
| ZrO2-Ce0.87La0.13O1.935 | 2.19 | 0.16 |
| Sample | SBET/m2·g−1 | Vp/cm3·g−1 | Dp/nm |
|---|---|---|---|
| ZrO2 nanoparticles | 35 | - | - |
| ZrO2-CeO2 | 84 | 0.31 | 3 |
| ZrO2-Ce0.87Y0.13O1.935 | 76 | 0.35 | 4 |
| ZrO2-Ce0.87La0.13O1.935 | 63 | 0.17 | 3 |
| Sample | (Ce 4d + Ln Xd)/Zr 3d | (Ce + Ln)/Zr 1/at.% |
|---|---|---|
| ZrO2-CeO2 | 0.65 | 0.20 |
| ZrO2-Ce0.87Y0.13O1.935 | 0.67 | 0.18 |
| ZrO2-Ce0.87La0.13O1.935 | 0.73 | 0.16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barroso-Bogeat, A.; Daza Raposo, I.; Blanco, G.; Pintado, J.M. Tuning the Integration Rate of Ce(Ln)O2 Nanoclusters into Nanoparticulated ZrO2 Supports: When the Cation Size Matters. Materials 2020, 13, 2818. https://doi.org/10.3390/ma13122818
Barroso-Bogeat A, Daza Raposo I, Blanco G, Pintado JM. Tuning the Integration Rate of Ce(Ln)O2 Nanoclusters into Nanoparticulated ZrO2 Supports: When the Cation Size Matters. Materials. 2020; 13(12):2818. https://doi.org/10.3390/ma13122818
Chicago/Turabian StyleBarroso-Bogeat, Adrián, Iván Daza Raposo, Ginesa Blanco, and José María Pintado. 2020. "Tuning the Integration Rate of Ce(Ln)O2 Nanoclusters into Nanoparticulated ZrO2 Supports: When the Cation Size Matters" Materials 13, no. 12: 2818. https://doi.org/10.3390/ma13122818