Photodegradation of Microcystin-LR Using Visible Light-Activated C/N-co-Modified Mesoporous TiO2 Photocatalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Photocatalyst
2.3. Characterization of Photocatalyst
2.4. Photocatalytic Experiments
3. Results and Discussion
3.1. Characteristics of Photocatalyst
3.2. Photocatalytic Activity of Photocatalyst
3.2.1. Degradation of MC-LR over TiO2 Photocatalyst
3.2.2. Improvement of Photocatalytic Degradation
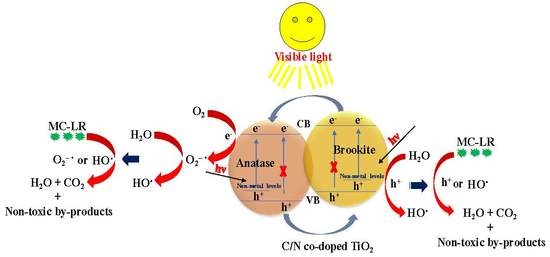
3.2.3. Proposed Mechanism for MC-LR Removal
(i) Adsorption:
(ii) Photodegradation:
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chae, S.; Noeiaghaei, T.; Oh, Y.; Kim, I.S.; Park, J.-S. Effective Removal of Emerging Dissolved Cyanotoxins from Water using Hybrid Photocatalytic Composites. Water Res. 2019, 149, 421–431. [Google Scholar] [CrossRef]
- Carmichael, W.W. The cyanotoxins. Adv. Bot. Res. 1997, 27, 211–256. [Google Scholar]
- Nawaz, M.; Moztahida, M.; Kim, J.; Shahzad, A.; Jang, J.; Miran, W.; Lee, D.S. Photodegradation of microcystin-LR using graphene-TiO2/sodium alginate aerogels. Carbohydr. Polym. 2018, 199, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Khadgi, N.; Upreti, A.R. Photocatalytic degradation of Microcystin-LR by visible light active and magnetic, ZnFe2O4-Ag/rGO nanocomposite and toxicity assessment of the intermediates. Chemosphere 2019, 221, 441–451. [Google Scholar] [CrossRef]
- Antoniou, M.G.; de la Cruz, A.A.; Dionysiou, D.D. Cyanotoxins: New Generation of Water Contaminants. J. Environ. Eng. 2005, 131, 1239–1243. [Google Scholar] [CrossRef]
- Guo, Q.; Li, H.; Zhang, Q.; Zhang, Y. Fabrication, characterization and mechanism of a novel Z-scheme Ag3PO4/NG/Polyimide composite photocatalyst for microcystin-LR degradation. Appl. Catal. B Environ. 2018, 229, 192–203. [Google Scholar] [CrossRef]
- Andersen, J.; Han, C.; O’Shea, K.; Dionysiou, D.D. Revealing the degradation intermediates and pathways of visible light-induced NF-TiO2 photocatalysis of microcystin-LR. Appl. Catal. B Environ. 2014, 154–155, 259–266. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Zhao, J.; Song, J.; Zhou, L.; Wang, J.; Tong, X.; Chen, Y. An alternative to in situ photocatalytic degradation of microcystin-LR by worm-like N, P co-doped TiO2/expanded graphite by carbon layer (NPT-EGC) floating composites. Appl. Catal. B Environ. 2017, 206, 479–489. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Khedr, T.M.; Zhang, G.; Vogiazi, V.; Ismail, A.A.; O’Shea, K.; Dionysiou, D.D. Tailored synthesis of anatase-brookite heterojunction photocatalysts for degradation of cylindrospermopsin under UV-Vis light. Chem. Eng. J. 2017, 310, 428–436. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Ismail, A.A.; Bahnemann, D.W. Photodegradation of 4-aminoantipyrine over Nano-Titania Heterojunctions Using Solar and LED Irradiation Sources. J. Environ. Chem. Eng. 2019, 17, 102797. [Google Scholar] [CrossRef]
- Feitz, A.J.; Waite, D.T.; Jones, G.J.; Boyden, B.H.; Orr, P.T. Photocatalytic degradation of the blue green algal toxin microcystin-LR in a natural organic aqueous matrix. Environ. Sci. Technol. 1999, 33, 243–249. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. A review on non-metal ion doped titania for the photocatalytic degradation of organic pollutants under UV/solar light: Role of photogenerated charge carrier dynamics in enhancing the activity. Appl. Catal. B Environ. 2013, 140–141, 559–587. [Google Scholar] [CrossRef]
- Hu, X.; Hu, X.; Tang, C.; Wen, S.; Wu, X.; Long, J.; Yang, X.; Wang, H.; Zhou, L. Mechanisms underlying degradation pathways of microcystin-LR with doped TiO2 photocatalysis. Chem. Eng. J. 2017, 330, 355–371. [Google Scholar] [CrossRef]
- Fiorenza, R.; Bellardita, M.; Scirè, S.; Palmisano, L. Effect of the addition of different doping agents on visible light activity of porous TiO2 photocatalysts. Mol. Catal. 2018, 455, 108–120. [Google Scholar] [CrossRef]
- Pedrosa, M.; Pastrana-Martínez, L.M.; Pereira, M.F.R.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M.T. N/S-doped graphene derivatives and TiO2 for catalytic ozonation andphotocatalysis of water pollutants. Chem. Eng. J. 2018, 348, 888–897. [Google Scholar] [CrossRef]
- Abdelraheem, W.H.M.; Patil, M.K.; Nadagouda, M.N.; Dionysiou, D.D. Hydrothermal synthesis of photoactive nitrogen- and boron- codoped TiO2 nanoparticles for the treatment of bisphenol A in wastewater: Synthesis, photocatalytic activity, degradation byproducts and reaction pathways. Appl. Catal. B 2019, 241, 598–611. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Álvarez Docio, C.M.; Zapata-Ramírez, V.; Fernández, J.F. Hierarchical nano ZnO-micro TiO2 625 composites: High UV protection yield lowering photodegradation in sunscreens. Ceram. Int. 2018, 44, 2827–2834. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Leret, P.; Álvarez Docio, C.M.; del Campo, A.; Fernández, J.F. Enhancement of UV absorption behavior in ZnO-TiO2 composites. Bol. Soc. Esp. Ceram. Vid. 2016, 55, 55–62. [Google Scholar] [CrossRef]
- Liu, G.; Han, C.; Pelaez, M.; Zhu, D.; Liao, S.; Likodimos, V.; Kontos, A.G.; Falaras, P.; Dionysiou, D.D. Enhanced visible light photocatalytic activity of C N-codoped TiO2 films for the degradation of microcystin-LR. J. Mol. Catal. A Chem. 2013, 372, 58–65. [Google Scholar] [CrossRef]
- Peng, G.; Fan, Z.; Wang, X.; Sui, X.; Chen, C. Photodegradation of microcystin-LR catalyzed by metal phthalocyanines immobilized on TiO2-SiO2 under visible-light irradiation. Water Sci. Technol. 2015, 72, 1824–1831. [Google Scholar] [CrossRef]
- He, X.; Pelaez, M.; Westrick, J.A.; O’Shea, K.E.; Hiskia, A.; Triantis, T.; Kaloudis, T.; Stefan, M.I.; de la Cruz, A.A.; Dionysiou, D.D. Efficient removal of microcystin-LR by UV-C/H2O2 in synthetic and natural water samples. Water Res. 2012, 46, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, J.; Tian, B.; Anpo, M. Tartaric acid-assisted preparation and photocatalytic performance of titania nanoparticles with controllable phases of anatase and brookite. J. Mater. Sci. 2012, 47, 5743–5751. [Google Scholar] [CrossRef]
- Nagase, T.; Ebina, T.; Iwasaki, T.; Hayashi, H.; Onodera, Y.; Chatterjee, M. Hydrothermal synthesis of brookite. Chem. Lett. 1999, 9, 911–912. [Google Scholar] [CrossRef]
- Yamashita, H.; Harada, M.; Misaka, J.; Takeuchi, M.; Neppolian, B.; Anpo, M. Photocatalytic degradation of organic compounds diluted in water using visible light-responsive metal ion-implanted TiO2 catalysts: Fe ion-implanted TiO2. Catal. Today 2003, 84, 91–196. [Google Scholar] [CrossRef]
- Shen, X.; Tian, B.; Zhang, J. Tailored preparation of titania with controllable phases of anatase and brookite by an alkalescent hydrothermal route. Catal. Today 2013, 201, 151–158. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Perego, C.; Clemençon, I.; Rebours, B.; Revel, R.; Durupthy, O.; Cassaignon, S.; Jolivet, J.-P. Thermal Stability of Brookite-TiO2 Nanoparticles with Controlled Size and Shape: In-situ studies by XRD. Mater. Res. Soc. Symp. Proc. 2009, 1146, NN04. [Google Scholar]
- Hu, Y.; Tsai, H.-L.; Huang, C.-L. Effect of brookite phase on the anatase–rutile transition in titania nanoparticles. J. Eur. Ceram. Soc. 2003, 23, 691–696. [Google Scholar] [CrossRef]
- Ye, X.S.; Sha, J.; Jiao, Z.K.; Zhang, L.D. Thermoanalytical characteristic of nanocrystalline brookite-based titanium dioxide. Nanostruct. Mater. 1997, 8, 919–927. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Robben, L.; Alkaim, A.; Bahnemann, D. Brookite versus anatase TiO2 photocatalysts: Phase transformations and photocatalytic activities. Photochem. Photobiol. Sci. 2013, 12, 602–609. [Google Scholar] [CrossRef]
- Allen, N.S.; Mahdjoub, N.; Vishnyakov, V.; Kelly, P.J.; Kriek, R.J. The effect of crystalline phase (anatase, brookite and rutile) and size on the photocatalytic activity of calcined polymorphic titanium dioxide (TiO2). Polym. Degrad. Stab. 2018, 150, 31–36. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Khedr, T.M.; Hakki, A.; Ismail, A.A.; Badawy, W.A.; Bahnemann, D.W. Visible Light Activated Carbon and Nitrogen Co-doped Mesoporous TiO2 as Efficient Photocatalyst for Degradation of Ibuprofen. Sep. Purif. Technol. 2017, 173, 258–268. [Google Scholar] [CrossRef]
- Gao, K. Strongly intrinsic anharmonicity in the low-frequency Raman mode in nanocrystalline anatase TiO2. Physica B 2007, 398, 33–37. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321. [Google Scholar] [CrossRef]
- Lin, H.; Li, L.; Zhao, M.; Huang, X.; Chen, X.; Li, G.; Yu, R. Synthesis of High-Quality Brookite TiO2 Single-Crystalline Nanosheets with Specific Facets Exposed: Tuning Catalysts from Inert to Highly Reactive. J. Am. Chem. Soc. 2012, 134, 8328–8331. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the Least Known TiO2 Photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Andino, J.M.; Li, Y. Bicrystalline TiO2 with controllable anatase–brookite phase content for enhanced CO2 photoreduction to fuels. J. Mater. Chem. A 2013, 1, 8209–8216. [Google Scholar] [CrossRef]
- Zhu, G.; Lin, T.; Lü, X.; Zhao, W.; Yang, C.; Wang, Z.; Yin, H.; Liu, Z.; Huang, F.; Lin, J. Black brookite titania with high solar absorption and excellent photocatalytic performance. J. Mater. Chem. A 2013, 1, 9650–9653. [Google Scholar] [CrossRef]
- Xu, J.; Wu, S.; Ri, J.H.; Jin, J.; Peng, T. Bilayer film electrode of brookite TiO2 particles with different morphology to improve the performance of pure brookite-based dye sensitized solar cells. J. Power Sources 2016, 327, 77–85. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Ismail, A.A.; Bahnemann, D.W. Highly Efficient Solar Light-Assisted TiO2 Nanocrystalline for Photodegradation of Ibuprofen Drug. Opt. Mater. 2019, 88, 117–127. [Google Scholar] [CrossRef]
- Jiang, R.; Zhu, H.-Y.; Chen, H.-H.; Yao, J.; Fu, Y.-Q.; Zhang, Z.-Y.; Xu, Y.-M. Effect of calcination temperature on physical parameters and photocatalytic activity of mesoporous titania spheres using chitosan/poly (vinyl alcohol) hydrogel beads as a template. Appl. Surf. Sci. 2014, 319, 189–196. [Google Scholar] [CrossRef]
- Robben, L.; Ismail, A.A.; Lohmeier, S.J.; Feldhoff, A.; Bahnemann, D.W.; Buhl, J.-C. Facile synthesis of highly ordered mesoporous and well crystalline TiO2: Impact of different gas atmosphere and calcinations temperature on structural properties. Chem. Mater. 2012, 24, 1268–1275. [Google Scholar] [CrossRef]
- Matsumoto, T.; Iyi, N.; Kaneko, Y.; Kitamura, K.; Ishihara, S.; Takasu, Y.; Murakami, Y. High visible-light photocatalytic activity of nitrogen-doped titania prepared from layered titania/isostearate nanocomposite. Catal. Today 2007, 120, 226–232. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.C.; Nadagouda, M.; Han, C.; O’Shea, K.; El-Sheikh, S.M.; Ismail, A.A.; Dionysiou, D.D. Visible light-sensitized S, N and C co-doped polymorphic TiO2 for photocatalytic destruction of microcystin-LR. Appl. Catal. B Environ. 2014, 144, 614–621. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, W.; Wu, Z. Characterization and photocatalytic activities of C, N and S co-doped TiO2 with 1D nanostructure prepared by the nano-confinement effect. Nanotechnology 2008, 19, 365–607. [Google Scholar] [CrossRef]
- Ma, D.; Xin, Y.; Gao, M.; Wu, J. Fabrication and photocatalytic properties of cationic and anionic S-doped TiO2 nanofibers by electrospinning. Appl. Catal. B Environ. 2014, 147, 49–57. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Zhang, G.; El-Hosainy, H.M.; Ismail, A.A.; O’Shea, K.E.; Falaras, P.; Kontos, A.G.; Dionysiou, D.D. High performance sulfur, nitrogen and carbon doped mesoporous anatase–brookite TiO2 photocatalyst for the removal of microcystin-LR under visible light irradiation. J. Hazard. Mater. 2014, 280, 723–733. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Hakki, A.; Ismail, A.A.; Badawy, W.A.; Bahnemann, D.W. highly active non-metals doped mixed-phase TiO2 for photocatalytic oxidation of ibuprofen under visible light. Photochem. Photobiol. A Chem. 2017, 346, 530–540. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Mesoporous titania photocatalysts: Preparation, characterization and reaction mechanisms. J. Mater. Chem. 2011, 21, 11686–11707. [Google Scholar] [CrossRef]
- Atitar, M.F.; Ismail, A.A.; Al-Sayari, S.A.; Bahnemann, D.; Afanasev, D.; Emeline, A.V. Mesoporous TiO2 nanocrystals as efficient photocatalysts: Impact of calcination temperature and phase transformation on photocatalytic Performance. Chem. Eng. J. 2015, 264, 417–424. [Google Scholar] [CrossRef]
- Leroy, P.; Tournassat, C.; Bizi, M. Influence of surface conductivity on the apparent zeta potential of TiO2 nanoparticles. J. Colloid Interface Sci. 2011, 356, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Suttiponparnit, K.; Jiang, J.; Sahu, M.; Suvachittanont, S.; Charinpanitkul, T.; Biswas, P. Role of Surface Area, Primary Particle Size, and Crystal Phase on Titanium Dioxide Nanoparticle Dispersion Properties. Nanoscale Res. Lett. 2011, 6, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Daou, I.; Chfaira, R.; Zegaoui, O.; Aouni, Z.; Ahlafi, H. Physico-Chemical Characterization and Interfacial Electrochemical Properties of Nanoparticles of Anatase-TiO2 Prepared by the Sol-Gel Method. Med. J. Chem. 2013, 2, 569–582. [Google Scholar]
- Zeng, M. Influence of TiO2 Surface Properties on Water Pollution Treatment and Photocatalytic Activity. Bull. Korean Chem. Soc. 2013, 34, 953–956. [Google Scholar] [CrossRef]
- Wang, P. Aggregation of TiO2 Nanoparticles in Aqueous Media: Effects of pH, Ferric Ion and Humic Acid. Int. J. Environ. Sci. Nat. Res. 2017, 1, 555575. [Google Scholar] [CrossRef]
- Wu, S.; Lv, J.; Wang, F.; Duan, N.; Li, Q.; Wang, Z. Photocatalytic degradation of microcystin-LR with a nanostructured photocatalyst based on upconversion nanoparticles@TiO2 composite under simulated solar lights. Sci. Rep. 2017, 7, 14435. [Google Scholar] [CrossRef] [PubMed]
- Zanjanchi, M.A.; Ebrahimian, A.; Arvand, M. Sulphonated cobalt phthalocyanine-MCM-41: An active photocatalyst for degradation of 2,4-dichlorophenol. J. Hazard. Mater. 2010, 175, 992–1000. [Google Scholar] [CrossRef]
- Kisch, H. On the Problem of Comparing Rates or Apparent Quantum Yields in Heterogeneous Photocatalysis. Angew. Chem. Int. Ed. 2010, 49, 9588–9589. [Google Scholar] [CrossRef]













| Samples | Crystal Size (nm) | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) | Band Gap (eV) | |
|---|---|---|---|---|---|---|
| A | B | |||||
| CDT-0.00 | 16 | 32 | 72.41 | 0.29 | 8.6 | 2.79 |
| CDT-300 | 17 | 35 | 66.00 | 0.27 | 9.3 | 2.82 |
| CDT-400 | 20 | 42 | 55.22 | 0.26 | 9.8 | 2.86 |
| CDT-500 | 25 | 50 | 20.51 | 0.23 | 10.7 | 3.00 |
| Samples | Assignment [Peak Position (cm−1)] | |
|---|---|---|
| Anatase | Brookite | |
| CDT-0.00 | Eg [145.5, 636], B1g [517.5], A1g [396, 517.5] | Ag [199, 636], A1g [396], B1g [517.5] |
| CDT-300 | Eg [146.5, 639], B1g [518], A1g [397, 518] | Ag [199, 639], A1g [397], B1g [323, 518] |
| CDT-400 | Eg [148.1, 641.5], B1g [518.1], A1g [399, 518.1] | Ag [199, 641.5], A1g [399], B1g [518.1] |
| CDT-500 | Eg [149, 644], B1g [521], A1g [400, 521] | Ag [199, 644], A1g [400], B1g [323, 521] |
| Samples | Content (at. %) | Ratio | |||
|---|---|---|---|---|---|
| Ti 2p | O 1s | C 1s | N 1s | O/Ti | |
| CDT-0.00 | 20.20 | 56.20 | 21.70 | 1.90 | 2.8 |
| CDT-300 | 20.64 | 57.60 | 20.10 | 1.66 | 2.8 |
| CDT-400 | 21.05 | 58.10 | 19.40 | 1.45 | 2.8 |
| CDT-500 | 21.98 | 58.60 | 18.20 | 1.22 | 2.7 |
| Vibration Mode | Bands Location (cm−1) |
|---|---|
| Ti-O; Ti-O-Ti (stretching) | 400–900 |
| O-H (stretching) | 3418 |
| O-H (bending) | 1630 |
| N-H (stretching) | 3700–3800 |
| N-H (bending) | 1630; 1416 |
| C-H (stretching) | 2922 |
| C-H (bending) | 1416 |
| Ti-N (bending) | 1416, 480 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khedr, T.M.; El-Sheikh, S.M.; Ismail, A.A.; Kowalska, E.; Bahnemann, D.W. Photodegradation of Microcystin-LR Using Visible Light-Activated C/N-co-Modified Mesoporous TiO2 Photocatalyst. Materials 2019, 12, 1027. https://doi.org/10.3390/ma12071027
Khedr TM, El-Sheikh SM, Ismail AA, Kowalska E, Bahnemann DW. Photodegradation of Microcystin-LR Using Visible Light-Activated C/N-co-Modified Mesoporous TiO2 Photocatalyst. Materials. 2019; 12(7):1027. https://doi.org/10.3390/ma12071027
Chicago/Turabian StyleKhedr, Tamer M., Said M. El-Sheikh, Adel A. Ismail, Ewa Kowalska, and Detlef W. Bahnemann. 2019. "Photodegradation of Microcystin-LR Using Visible Light-Activated C/N-co-Modified Mesoporous TiO2 Photocatalyst" Materials 12, no. 7: 1027. https://doi.org/10.3390/ma12071027