Development of a Zeolite A/LDH Composite for Simultaneous Cation and Anion Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of the Materials
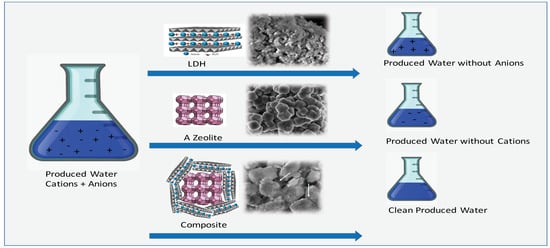
2.2. Treatment of Water Produced from Petroleum
3. Results
3.1. Synthesis and Characterization
3.2. Treatment of a Sample of Water Produced from Petroleum
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Srivatsa Bettahalli, N.M.; Chisca, S.; Khalid, M.K.; Ghaffour, N.; Vilagines, R.; Nunes, S.P. Polyoxadiazole hollow fibers for produced water treatment by direct contact membrane distillation. Desalination 2018, 432, 32–39. [Google Scholar] [CrossRef]
- Khalib, Z.; Verbeek, P. (Shell) Water to Value—Produced Water Management for Sustainable Field Development of Mature and Green Fields. In Proceedings of the SPE International Conference on Health, Safety and Environment in Oil and Gas Exploration and Production, Kuala Lumpur, Malaysia, 20–22 March 2002; p. 4. [Google Scholar]
- Arthur, D.J.; Hochheiser, W.H.; Bottrell, M.D.; Brown, A.; Candler, J.; Cole, L.; DeLao, D.; Dillon, L.W.; Drazan, D.J.; Dusseault, M.B.; et al. Management of Produced Water from Oil and Gas Wells; NCP North American Resource Development: Washington, DC, USA, 2011. [Google Scholar]
- BR, H.; SR, D. Review of Potential Technologies for the Removal of Dissolved Components from Produced Water. Chem. Eng. Res. Des. 1994, 72, 176–188. [Google Scholar]
- Farhadian, M.; Duchez, D.; Vachelard, C.; Larroche, C. Monoaromatics removal from polluted water through bioreactors—A review. Water Res. 2008, 42, 1325–1341. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Chandran, P. Microbial Degradation of Petroleum Hydrocarbon Contaminants: An Overview; Hindaw: NewYork, NY, USA, 2011; Volume 2011, pp. 1–13. [Google Scholar]
- Guidelines Establishing Test Procedures for the Analysis of Oil and Grease and Non-Polar Material under the Clean Water Act and Resource Conservation and Recovery Act; Final Rule; Environmental Protection Agen: Washington, DC, USA, 1999; Volume 64, pp. 26315–26327.
- Resolução CONAMA no 357, de 17 de março de 2005; Ministério do Meio Ambiente: Brasília, Brasil, 2005; 23p.
- Resolução CONAMA no 430, de 13 de maio de 2011; Ministério do Meio Ambiente: Brasília, Brasil, 2011; pp. 1–8.
- Tellez, G.T.; Nirmalakhandan, N.; Gardea-Torresdey, J.L. Performance evaluation of an activated sludge system for removing petroleum hydrocarbons from oilfield produced water. Adv. Environ. Res. 2002, 6, 455–470. [Google Scholar] [CrossRef]
- Yamada, H.; Watanabe, Y.; Hashimoto, T.; Tamura, K.; Ikoma, T.; Yokoyama, S.; Tanaka, J.; Moriyoshi, Y. Synthesis and characterization of Linde A zeolite coated with a layered double hydoxide. J. Eur. Ceram. Soc. 2006, 26, 463–467. [Google Scholar] [CrossRef]
- Da Silva, L.N.; dos Santos Moraes, D.; Santos, S.C.A.; Corrêa, J.A.M. Joint synthesis of Zeolite A-LDH from mineral industry waste. Appl. Clay Sci. 2018, 161, 163–168. [Google Scholar] [CrossRef]
- Braga, A.A.C.; Morgon, N.H. Descrições estruturais cristalinas de zeólitos. Quim. Nova 2007, 30, 178–188. [Google Scholar] [CrossRef] [Green Version]
- Guisnet, M.; Ribeiro, F.R. Zeólitos: Um nanomundo ao serviço da catálise; Fundação Calouste Gulbenkian: Lisbon, Portugal, 2004; ISBN 9789723110715. [Google Scholar]
- Melo, C.R.; Riella, H.G. Síntese de zeólita tipo NaA a partir de caulim para obtenção de zeólita 5A através de troca iônica. Cerâmica 2010, 56, 340–346. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Gomes, J.F.P.; Puna, J.F.B.; Gonçalves, L.M.; Bordado, J.C.M. Study on the use of MgAl hydrotalcites as solid heterogeneous catalysts for biodiesel production. Energy 2011, 36, 6770–6778. [Google Scholar] [CrossRef] [Green Version]
- Verified Syntheses os Zeolitic Materials; Mintova, S.; Barrier, N. (Eds.) Synthesis Commission of the International Zeolite Association, 2016; ISBN 978-0-692-68539-6. [Google Scholar]
- Climent, M.J.; Corma, A.; Iborra, S.; Epping, K.; Velty, A. Increasing the basicity and catalytic activity of hydrotalcites by different synthesis procedures. J. Catal. 2004, 225, 316–326. [Google Scholar] [CrossRef]
- Fraccarollo, A.; Cossi, M.; Marchese, L. DFT simulation of Mg/Al hydrotalcite with different intercalated anions: Periodic structure and solvating effects on the iodide/triiodide redox couple. Chem. Phys. Lett. 2010, 494, 274–278. [Google Scholar] [CrossRef]
- Radha, A.V.; Kamath, P.V.; Shivakumara, C. Mechanism of the anion exchange reactions of the layered double hydroxides (LDHs) of Ca and Mg with Al. Solid State Sci. 2005, 7, 1180–1187. [Google Scholar] [CrossRef]
- Collection of Simulated XRD Powder Patterns for Zeolites; Treacy, M.M.J.; Higgins, J.B. (Eds.) Elsevier: Amsterdam, The Netherlands, 2001; ISBN 978-0-444-53067-7. [Google Scholar]
- Maia, A.Á.B.; Neves, R.F.; Angélica, Rô.S.; Pöllmann, H. Synthesis, optimisation and characterisation of the zeolite NaA using kaolin waste from the Amazon Region. Production of Zeolites KA, MgA and CaA. Appl. Clay Sci. 2015, 108, 55–60. [Google Scholar] [CrossRef]
- Rocha Junior, C.A.F.; Santos, S.C.A.; Souza, C.A.G.; Angélica, R.S.; Neves, R.F. Síntese de zeólitas a partir de cinza volante de caldeiras: Caracterização física, química e mineralógica. Cerâmica 2012, 58, 43–52. [Google Scholar] [CrossRef]
- Seftel, E.M.; Niarchos, M.; Mitropoulos, C.; Mertens, M.; Vansant, E.F.; Cool, P. Photocatalytic removal of phenol and methylene-blue in aqueous media using TiO2@LDH clay nanocomposites. Catal. Today 2015, 252, 120–127. [Google Scholar] [CrossRef]
- Pesic, L.; Salipurovic, S.; Markovic, V.; Vucelic, D.; Kagunya, W.; Jones, W. Thermal characteristics of a synthetic hydrotalcite-like material. J. Mater. Chem. 1992, 2, 1069. [Google Scholar] [CrossRef]
- Nascimento, C.R. Síntese da zeólita A utilizando diatomita como fonte de sílicio e alumínio. Cerâmica 2014, 60, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Forano, C.; Hibino, T.; Leroux, F.; Taviot-Guého, C. Layered Double Hydroxides. In Handbook of Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2006; Volume 1, pp. 1021–1095. ISBN 1572-4352. [Google Scholar]
- Theiss, F.L.; Palmer, S.J.; Ayoko, G.A.; Frost, R.L. Sulfate intercalated layered double hydroxides prepared by the reformation effect. J. Therm. Anal. Calorim. 2012, 107, 1123–1128. [Google Scholar] [CrossRef]
- Xu, J.; Song, Y.; Zhao, Y.; Jiang, L.; Mei, Y.; Chen, P. Chloride removal and corrosion inhibitions of nitrate, nitrite-intercalated Mg–Al layered double hydroxides on steel in saturated calcium hydroxide solution. Appl. Clay Sci. 2018, 163, 129–136. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kushwaha, P.K.; Srivastava, V.K.; Bhatt, S.D.; Jasra, R.V. Effect of hydrothermal conditions on structural and textural properties of synthetic hydrotalcites of varying Mg/Al ratio. Ind. Eng. Chem. Res. 2007, 46, 4856–4865. [Google Scholar] [CrossRef]






| Material | Al2O3 | MgO | SiO2 | Na2O | Si/Al | Mg/Al |
|---|---|---|---|---|---|---|
| Zeolite A | 35.50 | 1.5 | 45.35 | 16.9 | 1.10 | 0.05 |
| LDH | 37.97 | 58.21 | 0 | 3.06 | 0 | 1.9 |
| ZAHD | 34.71 | 20.9 | 33.96 | 10.0 | 0.83 | 0.77 |
| Material | SBET (m2/g) | VTP (cm3/g)/0.98 |
|---|---|---|
| Zeolite A | 5 | 0.01 |
| LDH | 113 | 0.60 |
| ZAHD | 57 | 0.21 |
| Parameters | Initial Concentration on PW (mg/L) | ZEO (mg/L) | LDH (mg/L) | LDH/ZEO (mg/L) | ZEO/LDH (mg/L) | COMPOSITE (mg/L) |
|---|---|---|---|---|---|---|
| Al | 3.913 | 0 | 3.930 | 0.078 | 0 | 0 |
| Cd | 0.448 | 0 | 0.446 | 0 | 0 | 0.059 |
| Cu | 0.385 | 0 | 0.384 | 0 | 0 | 0 |
| Mn | 2.319 | 0.070 | 2.322 | 0.105 | 0.075 | 0.740 |
| Ni | 0.482 | 0 | 0.484 | 0 | 0 | 0.131 |
| Pb | 0.437 | 0 | 0.435 | 0 | 0 | 0 |
| Zn | 2.407 | 0 | 0.404 | 0 | 0 | 0.058 |
| Chloride | 203.971 | 200.345 | 32.965 | 25.276 | 28.965 | 29.138 |
| Bromide | 18.656 | 18.530 | 3.572 | 2.982 | 3.572 | 8.09 |
| nitrite | 13.97 | 14.02 | 2.980 | 2.947 | 2.98 | 6.15 |
| nitrate | 60.757 | 60.573 | 10.239 | 9.908 | 7.239 | 13.731 |
| phosphate | 18.304 | 18.132 | 2.080 | 2.019 | 2.08 | 0.07 |
| sulphate | 19.027 | 19.123 | 14.247 | 10.406 | 10.647 | 5.679 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezerra, B.G.P.; Bieseki, L.; da Silva, D.R.; Pergher, S.B.C. Development of a Zeolite A/LDH Composite for Simultaneous Cation and Anion Removal. Materials 2019, 12, 661. https://doi.org/10.3390/ma12040661
Bezerra BGP, Bieseki L, da Silva DR, Pergher SBC. Development of a Zeolite A/LDH Composite for Simultaneous Cation and Anion Removal. Materials. 2019; 12(4):661. https://doi.org/10.3390/ma12040661
Chicago/Turabian StyleBezerra, Breno Gustavo Porfírio, Lindiane Bieseki, Djalma Ribeiro da Silva, and Sibele Berenice Castellã Pergher. 2019. "Development of a Zeolite A/LDH Composite for Simultaneous Cation and Anion Removal" Materials 12, no. 4: 661. https://doi.org/10.3390/ma12040661