3.1. X-Ray Diffraction (XRD) Analysis and N2 Physisorption at 77 K
Figure 1A shows the XRD spectra of TiO
2_A, TiO
2_B, and the P-25 samples, all featuring the diffraction peaks of anatase (a). No additional peaks are seen, therefore, indicating (a) the only presence of anatase. Converseley, the XRD pattern of P-25 shows the presence of both anatase and rutile (70:30 anatase to rutile ratio).
As is known, the anatase phase is more effective than the rutile in terms of photocatalytic activity, due to several textural and structural factors. In particular, the electron migration from the bulk to the surface in the anatase phase is faster than in the rutile phase, and for this reason, the recombination rate is lower [
49]. However, the anatase-rutile mixed phase may provide a synergistic effect towards several photocatalytic reactions, especially when the two phases stay in close contact.
Moreover, the presence of both rutile and anatase phases enhances the photocatalytic activity since the intimate contact may favour the electron migration from rutile (conduction band) to anatase (trapping sites below the conduction band of rutile). This effect reduces the recombination rate in the rutile [
49,
50,
51].
Figure 1B shows the magnification of the main peak in the spectra in the 2
θ range of 24–27°. It is observed that the peaks in the spectra of TiO
2_A and TiO
2_B are shifted to a relatively higher 2
θ with respect to that in the spectrum of P-25. Such shifts may reflect the structural differences among the samples (in terms of cell parameter). For example, it is possible to observe a general trend of the 2
θ shift with the crystallite size calculated by the Scherrer’s equation (D
c), as shown in
Table 1. As a whole, it appears that the smaller D
c values the lower the 2
θ values.
Figure 1C summarizes the XRD spectra of the doped titania samples. The lattice is characterized by only the presence of anatase. Moreover, no segregated phases (i.e., zirconium or phosphorus oxides) were observed in the XRD spectra of these samples, thus suggesting that the dopants are effectively dispersed and incorporated in the solid framework.
Figure 1D shows the magnification of the main peak in the 2
θ range of 24–27°. In the spectrum of Zr-TiO
2 (blue curve), the peak seems shifted to a lower 2
θ (signal at 25.11°) with respect to the peak position of TiO
2_B (signal at 25.35°). This shift may originate from the incorporation of the dopant in the titania structure. On the other hand, in the P-TiO
2 spectrum the peak is shifted to a lower 2
θ with respect to that of TiO
2_A.
The dopants into the titania framework may lead to different cell volumes and lattice parameters. We observed that TiO
2 doped systems exhibit different trends as compared with those of the TiO
2 samples. Specifically, the ionic radii of Zr
4+ and Ti
4+ cations are about 72 pm and 75 pm, respectively, thus explaining the easy metal substitution in the lattice and the similar crystallite sizes. On the other hand, for the P-TiO
2 sample, it is possible to observe a change in the crystallite size (smaller values) if the phosphorus ions (52 pm) are introduced to the TiO
2 lattice.
Table 1 reports the crystallite sizes (
DC) for the samples, as calculated by the Scherrer’s equation.
As a whole, particles with lower Dc values have a higher probability to have aboundant edges and corners on the solid surface as compared with the larger ones, for instance, that have higher amounts of terrace sites [
52]. Due to the presence of these surface defects (i.e., edges and corners), the electron valence band shifts toward higher values because the local electron valence band on the surface increases. This phenomenon, along with the co-presence of the instauration, allows the absorption energy of atoms or molecules on the catalyt surface to increase. This means a major affinity between the catalyst surface and the molecules [
53].
Table 1 also reports the results derived from the N
2 physisorption at 77 K. The BET surface areas for the samples show an increasing trend according to the following order:
It appears that smaller crystallites lead to higher SBET values (e.g., P-TiO2). The two pure titania samples (TiO2_A and TiO2_B) have different SBET values and this is possibly due to the different synthesis procedures. During the synthesis of the TiO2_A, the hydrothermal treatment in the autoclave was carried out for 48 h. This aging step is crucial for the growth of meso-structures in the sample. In fact, this material is characterized by high porosity and surface area. Conversely, the synthesis of the TiO2_B does not include the aging step and then worse textural properties occur
3.2. FESEM and Energy Dispersive X-Ray Analysis (EDX)
Figure 2 shows the FESEM images of the prepared samples. The two pure titania samples exhibit the presence of nanoparticles (in the range 10–20 nm) agglomerated in clusters. On the other hand, the specific surface areas for these materials depend on both their textural and structural properties, as well the formation of the interparticle cavities (self-assembled NPs) may have a role on the SSA. Conversely, the XRD analysis (Scherrer’s equation) confirms the presence of different crystallite sizes for the prepared materials (see
Table 1), specifically, it appears that the lower the crystallites size the higher the SSA.
The P-TiO
2 sample is comprised of larger particles as compared with pure titania-based catalysts, whereas, the Zr-TiO
2 has the most compact structure among the synthesized samples. For comparison purposes, the image of the P-25 sample was also added in
Figure 2. In this case, this material is comprised of larger particles as compared with the others.
The distribution of the particle sizes was carried out by using the ImageJ software [
54]. For each sample, 60 particles were considered.
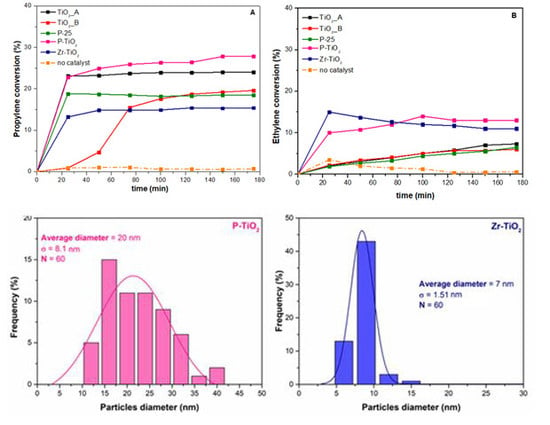
Figure 3 reports the histogram of the particle size distribution, as well as the average particle diameter and the deviation standard (σ).
The average diameters of the TiO
2_A and of TiO
2_B samples are about 15 nm and 16 nm, respectively. The particle size distribution for the two samples is also comparable. Despite the resemblance of the morphology, the two samples have different surface areas (
Section 3.1), with TiO
2_B having the lowest surface area. This means that TiO
2_A is comprised of larger pores inside the structure and rather than those between the particles (interparticle porosity).
The P-TiO2 sample is comprised of larger particles, with an average diameter of about 20 nm. This means that when P is incorporated in the TiO2 structure there is the formation of larger particles.
Smaller particles are observed with the Zr-TiO2 sample (approximately 7 nm). In fact, this sample exhibits a compact structure, as observed by FESEM. Despite its compactness, it exhibits a high surface area (136 m2·g−1). It is likely that the sample possess a high degree of intraparticle porosity, which crucially contributes to the overall porosity of the catalyst.
The P-25 sample shows the largest average diameter in the set of samples (about 30 nm). Nevertheless, this sample has the lowest surface area. Therefore, it appears that P-25 have a lower intraparticle porosity as compared with, for example, with Zr-TiO2.
Table 2 summarizes the elemental composition of the doped titania samples derived from the EDX analysis. The atomic percentage of O is 70% for both samples. The percentage of Ti is 28% for P-TiO
2 and 26% for Zr-TiO
2. Through these values, it is possible to evaluate the relative abundance of P and Zr to verify the dopant-to-Ti ratio (P/Ti and Zr/Ti). It was found that the P/Ti is about 5 at%, while the Zr/Ti is close to 18 at%. This confirms the theoretical dopant loading expected prior to the synthesis.
Figure 4 and
Figure 5 report the EDX maps for the doped samples. The elemental mapping is a useful method to analyze the distribution of the elements in the sample. Both dopants, Zr (
Figure 4, light blue) and P (
Figure 5, violet) seem to be effectively distributed in their respective samples. This indicates the benefit of using the adopted techniques for maintaining a good dopant distribution in the samples.
3.3. (DR)UV-Vis Spectroscopy
Figure 6 reports the (DR)UV-Vis spectra of the studied samples.
Compared to P-25, the TiO
2_A and TiO
2_B samples absorb radiation in a broader range of wavelength (i.e., this absorption occurs at 425 and 350 nm, as shown by the inset on
Figure 6A). The absorption by the TiO
2_A sample is observed at 375 and 330 nm, due to charge transfer (CT) transitions in the solid.
Figure 6B shows the corresponding Tauc’s plot, used to evaluate the band gap energies of the samples which are summarized in
Table 3 with their absorption edge wavelength. The values of λ are also reported in
Table 3.
Among the pure titania samples, TiO2_A has the highest value of band gap energy (approximately 3.17 eV), while Zr-TiO2 has the lowest value, 2.96 eV. From the point of view of band gap energy, the most potentially active catalyst seems to be Zr-TiO2 because the energy required to promote electrons from the valence to conduction band is the lowest.
Similarly,
Figure 6C shows the (DR)UV-Vis spectra of the doped titania samples. The Zr-TiO
2 sample has a range of absorption in the visible light region, as evidenced by the inset in
Figure 6C.
Figure 6D shows the corresponding Tauc’s plot of the spectra and the band gap energy evaluated (these values are reported in
Table 3 with the absorption wavelength edge of the samples).
3.4. X-ray Photoelectron Spectroscopy (XPS)
Table 4 shows the surface atomic percentage of the elements on the catalysts analyzed via XPS. In most cases, the Ti-to-O ratio is about one to three, lower than the theoretical ratio for a pure TiO
2 (one to two). This means that the catalysts have a higher oxygen abundance on the surface than in the bulk. The Ti-to-O ratio for Zr-TiO
2 seems lower than the average value, nevertheless, the slightly lower Ti quantity is actually compensated by a small percentage of Zr.
Figure 7 shows the Ti 2
p XP spectra of all the samples, which demonstrate a high-intensity spin-orbit peak in the 2
p3/2 region (average binding energy (BE) = 458.6 eV) and a low-intensity peak in the 2
p1/2 region, with a spin orbit splitting (Δ) of about 5.7 eV. All the spectra of the samples, regardless of the dopant, demonstrate a typical Ti 2
p spectrum of Ti
4+ in TiO
2 [
55]. Two peak components have been deconvoluted in the Ti 2
p3/2 region. The former centered at about 458.6 eV is ascribed to Ti
4+ while the latter centered at around 457.1 eV is assigned to Ti
3+ [
55].
Table 5 summarizes the relative abundances of Ti species on the surface of the catalysts. In general, the abundance of Ti
3+ on the surface is much lower than that of Ti
4+. Apparently, Zr-TiO
2 has the highest Ti
3+ abundance among the samples and this is most likely caused by the weakening of Ti–O–Ti bond induced by cation substitution with Zr, which has a larger ionic radius (0.75 and 0.61 Å for Zr
4+ and Ti
4+, respectively) [
55].
Figure 8 shows the O 1
s XP spectra of the samples. In most cases, two peaks corresponding to the Ti-O (lattice-like oxygen species bonded with Ti) and the non-lattice oxygen (NLO) species, related to defect sites such as hydroxyls (O–H), were identified at 531 eV and 529 eV, respectively [
56,
57,
58]. In the case of P-TiO
2, two NLO species were identified at 530.9 eV and 533.1 eV and they were associated with oxygen from phosphate and hydroxyls, respectively [
55].
Table 6 reports the relative abundances of both species evaluated through the curve fitting of the XP spectra. It is worth noting that the doped samples have a higher abundance of NLO species on the surface (38% for Zr-doped and 36% for P-doped sample) than the pure titania ones. The higher abundance of such species, such as OH groups on the surface, may bestow on the doped samples an improved ability to mediate VOC abatement. This enhancement may be because the hydroxyl groups on the surface are transformed via oxidation to the OH radicals, which may participate in the photocatalytic VOC oxidation [
59].
Figure 9 shows the P 2
p and Zr 3
d XP spectra of P-TiO
2 and Zr-TiO
2, respectively. The relative abundances of these dopants with respect to the constituting elements of the parent oxide (i.e., Ti and O) are reported in
Table 4. The percentage of P on the surface of the P-TiO
2 sample is about 3 at%, while the percentage of Zr on the surface of Zr-TiO
2 sample is around 4 at%, as shown in
Table 4. From the XPS analysis, the P-TiO
2 sample has about 3 at% of P on the surface, while from the EDX analysis the sample was observed to have about 2 at% of P in the bulk. This means that the dopant is more concentrated on the TiO
2 surface than in the bulk. Zr has the same atomic percentage in the bulk of Zr-TiO
2 as well as on the surface. The P 2
p spectrum of P-TiO
2 sample shows a peak at 132.3 eV, which corresponds to phosphate bonded to Ti [
55].
The spin orbit peak of Zr 3
d in the 3
d5/2 region is centered at 182.2 eV, which is simply ascribed to Zr
4+ [
55]. In the pure titania samples, there are no differences in the titanium and oxygen compositions, which are 25 at% and 75 at%, respectively, in all cases, as shown in
Table 4. When the dopant was inserted in the TiO
2 framework, the abundance of titanium and oxygen changes. The titanium abundance in P-TiO
2 is the same as that in pure TiO
2, while the oxygen abundance decreases from 75 at% to 72 at%. In Zr-TiO
2 the titanium abundance increases from 75 at% to 77 at%, while the oxygen abundance decreases from 25 at% to 19 at%.
3.5. Photocatalytic Activity
In the photocatalytic reaction, the electrons in the TiO
2 structure are promoted to the anatase conduction band (CB) and the corresponding holes are created in the valence band (VB). During this phenomenon, the ·OH and ·O
2− species are produced, according to the following Equations (1)–(4) [
60]:
These radical species additionally react with ethylene (5–8) to produce CO
2 and water vapor, represented in the following equations:
A similar reaction mechanism is observed for the photocatalytic oxidation of propylene (9–12):
Figure 10 shows the conversion of propylene (10A) and ethylene (10B) achieved during the photocatalytic tests. For comparison purposes, the conversion of the uncatalyzed reaction has also been included in
Figure 10 and it is represented by the orange curve.
Table 7 summarizes the VOC conversion values for all the catalysts.
As a whole, the catalytic performances (in terms of conversion) of the prepared samples is summarized as follows:
The most performing catalyst for both oxidation reactions appears to be the P-TiO2 (evaluated with TOS = 3 h). In fact, the propylene conversion achieved over this catalyst was about 27.8%, while the conversion of ethylene was close to 13%.
In the case of propylene oxidation (
Figure 10A), the two meso-structured samples (P-TiO
2 and TiO
2_A) seem to give a higher catalytic activity towards the reaction than the other samples. This suggests that the propylene oxidation reaction is textural dependent on these catalysts. The activity of the reaction is affected by the catalyst structure and morphology. On the basis of the previous N
2 physisorption and FESEM analyses, the meso-structure in the two samples brings about high surface area and large pore size. Propylene is a bulkier molecule than ethylene. Therefore, it is easily adsorbed on the catalyst surface, the diffusion limitation must be minimized, and the meso-structure helps tackle such a diffusional barrier.
The commercial sample, P-25, despite being relatively less active than the two meso-structured samples, induced a fast light-off reaction. From
Figure 10A, we observed that the conversion increased quickly to about 18% in the first few minutes. Although the sample is not morphologically endowed, the synergism between rutile and anatase might contribute to the initial catalytic activity.
The effect of dopant seems variable in the propylene oxidation. While phosphorus gives a beneficial effect on catalyst morphology and, subsequently, on catalytic activity, zirconium seems to disfavor this effect. This finding may be linked to its effect on morphology, as the incorporation of Zr, according to the N2 physisorption results (vide supra), leads to higher surface area but lower porosity. The contribution of surface area was appreciable in the first few minutes of the illumination, where the reaction ignited very quickly over Zr-TiO2 than over TiO2. However, the reaction proceeded more slowly afterwards as it was limited by the diffusion phenomena (due to smaller pore size).
In the case of ethylene oxidation (
Figure 10B), the P-TiO
2 and Zr-TiO
2 catalysts exhibited the highest conversion, whereas, the titania samples showed lower but comparable performances. Despite being equally active, the two most performing catalysts differ in morphology. This means that the ethylene oxidation reaction is not textural dependent on these samples. Variations in catalyst morphology hardly affect the catalytic performance. This is reasonable because ethylene is a simpler molecule than propylene and its smaller size should not be constrained by diffusion limitation. However, it seems that the reaction is affected by the presence of dopants. These foreign elements are traditionally introduced to titania lattice to narrow the band gap energy and, subsequently, to expand the absorption spectrum. The variation in band gap energy among the samples, as summarized in
Table 3, is, nevertheless, minor and UV irradiation is dominant. This means that another effect coming from the dopant plays a more important role in the catalysis and that could be the generation of defect sites on the surface. The XPS analysis (vide supra) has highlighted the increased quantity of OH groups, which are indicative of defect sites, on the surface of the doped titania samples.
Finally, a fairer comparison of catalytic activity among the catalysts was carried out by analyzing their specific reaction rates calculated at the end of the TOS (3 h). The rates were normalized with respect to catalyst weight (µmol h−1 gcat−1), as well as to specific surface area (µmol h−1 m−2). The latter specific rate eventually referred to the catalyst intrinsic activity.
Figure 11A,B summarize the values of the specific rates of propylene and ethylene oxidation for all catalysts normalized with respect to the catalyst weight. As we previously observed, the most active catalysts for propylene oxidation, according to their specific reaction rates, were P-TiO
2 and TiO
2_A (specific reaction rates = 1668 and 1440 µmol h
−1 g
−1, respectively) and those for ethylene oxidation were P-TiO
2 and Zr-TiO
2 (32.81 and 27.76 µmol h
−1 g
−1). For propylene oxidation, the trend for the specific reaction rate is in accordance with the one observed for the specific surface area (153 m
2 g
−1 for P-TiO
2 and 128 m
2 g
−1 for TiO
2_A, the highest in the series). The Zr-TiO
2 sample has the lowest specific reaction rate (approximately 924 µmol h
−1 g
−1) despite its high surface area (136 m
2 g
−1). The reason could be the morphology of this sample, i.e., the small particle dimension and the compactness reduces the accessibility of propylene to the active sites. For ethylene oxidation, the two most active catalysts are among those that have the highest surface area. However, the activity seems to be more affected by the presence of dopants (i.e., P and Zr), which likely modifies the surface chemical and electronic properties of the catalysts and plays a key role in the reaction mechanism.
Finally,
Figure 11C,D summarizes the values of the specific rates of propylene and ethylene oxidation for all catalysts normalized with respect to the catalyst surface area. The two low-surface area catalysts (TiO
2_B and P-25) were apparently the most intrinsically active catalysts for both oxidation reactions. From the XRD and FESEM micrography analysis, the two samples have the highest average particle diameters and average crystallite sizes (D
c). Moreover, in the case of TiO
2_B and P-25, the average particle diameter is equal to the average D
c. This means that the two samples are largely comprised of monocrystalline particles, which likely contain fewer bulk defects. It is widely believed that the presence of structural defects, regardless of their position in the catalyst (i.e., in the bulk or on the surface), affect the overall photocatalytic activity. Bulk defects are known to act as recombination centers for electron-hole pairs, whereas, surface defect sites act as traps for the photogenerated electrons [
61,
62,
63,
64]. While the former negatively impacts the photocatalytic activity, the latter may positively contribute to the photocatalytic activity as they extend the lifetime of holes. Fewer bulk defects in P-25 and TiO
2_B, due to the predominance of monocrystalline particles, may inhibit the electron-hole recombination. It is also known that for single crystal nanoparticles, smaller particles likely contain more edge and corner sites on the surface while larger particles contain more terrace sites (hence less surface defect sites) [
46]. Since both P-25 and TiO
2_B are characterized by larger particles, it is surmised that the two samples have fewer surface defect sites, thus a lower possibility of electron trapping.