Toxicological Evaluation of Luminescent Silica Nanoparticles as New Drug Nanocarriers in Different Cancer Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
2.3. Synthesis of Polyethylene Glycol (PEG) Derivatives
Synthesis of PEG(3000)-Silane (PEG1) and TsO-PEG(8000)-Silane (PEG2)
2.4. Synthesis of Luminescent Mesoporous Silica Nanoparticles
2.4.1. Synthesis and Functionalization of Cadmium Telluride Quantum Dots @ Mesoporous Silica Nanoparticles (CdTeQDs@MNs, CdTeQDs@MNs@PEG1)
2.4.2. Synthesis and Functionalization of Modified Luminescent Silicon Quantum Dots Coated with Mesoporous Silica Nanoparticles (SiQDs@Isoc@MNs, SiQDs@Isoc@MNs@PEG2)
2.5. Toxicological Studies
2.5.1. Cell Culture
2.5.2. Cell Viability Assays
MTT Reduction Assay
NR Internalization Assay
Lactate Dehydrogenase (LDH) Release
2.5.3. Statistical Analysis
2.6. Protein Encapsulation Studies
2.7. Doxorubicin Loading and In Vitro Release Studies
3. Results and Discussion
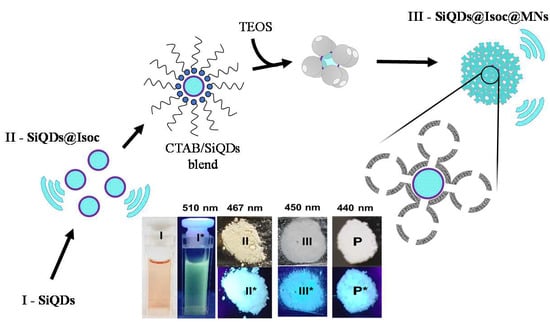
3.1. Synthesis and Characterization of Luminescence Mesoporous Silica Nanoparticles
3.2. In Vitro Toxicological Studies to Characterize the Toxicity Profile and Respective Mechanisms of the Different Nanomaterials
3.3. Encapsulation and Release Studies
3.4. Doxorubicin Loading and Release In Vitro Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, Z.; Deng, P.; Tang, S.; Kuang, D.; Zhang, F.; Li, J. Preparation of 2D molecularly imprinted materials based on mesoporous silicas via click reaction. J. Mater. Chem. B 2014, 2, 8418–8426. [Google Scholar] [CrossRef]
- Wilcoxon, J.P.; Samara, G.A. Tailorable, visible light emission from silicon nanocrystals. Appl. Phys. Lett. 1999, 74, 3164–3166. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; He, Y.; Chang, J.; Wang, L.; Chen, H.; Tan, Y.-W.; Wang, H.; Shao, Z. Surface-Modified Silicon Nanoparticles with Ultrabright Photoluminescence and Single-Exponential Decay for Nanoscale Fluorescence Lifetime Imaging of Temperature. J. Am. Chem. Soc. 2013, 135, 14924–14927. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ye, D.-X.; Liang, G.-H.; Chang, J.; Kong, J.-L.; Chen, J.-Y. One-step synthesis of water-dispersible silicon nanoparticles and their use in fluorescence lifetime imaging of living cells. J. Mater. Chem. B 2014, 2, 4338–4345. [Google Scholar] [CrossRef]
- Li, D.; He, X.; Chen, Y.; Li, W.; Zhang, Y. Novel Hybrid Structure Silica/CdTe/Molecularly Imprinted Polymer: Synthesis, Specific Recognition, and Quantitative Fluorescence Detection of Bovine Hemoglobin. ACS Appl. Mater. Interfaces 2013, 5, 12609–12616. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, A.; Gerion, D.; Visconte, M.; Sun, J.; Schwartzberg, A.; Chen, S.; Zhang, J.Z. Silica-coated CdTe quantum dots functionalized with thiols for bioconjugation to IgG proteins. J. Phys. Chem. B 2006, 110, 5779–5789. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cao, Y.; Chen, P.; Zhao, Q.; Shen, G. Fabrication od mesoporous CdTe/ZnO@SiO2 core/shell nanostructures with tunable dual emission and ultrasensitive flourescence response to metal ions. Chem. Mater. 2009, 21, 68–77. [Google Scholar] [CrossRef]
- Hu, X.; Zrazhevskiy, P.; Gao, X. Encapsulation of Single Quantum Dots with Mesoporous Silica. Ann. Biomed. Eng. 2010, 37, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Huo, D.; Hou, C.; Yang, M.; Fa, H.; Xia, C.; Chen, M. Mesoporous silica-coated quantum dots functionalized with folic acid for lung cancer cell imaging. Anal. Methods 2015, 7, 9649–9654. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.; Yang, R.H.S.; Yang, C.-S.; Lin, C.-H.; Chang, L.W. Toxicology and Biosafety Evaluations of Quantum Dots. In Nanotechnologies for the Life Sciences; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Yu, Y.; Rowland, C.E.; Schaller, R.D.; Korgel, B.A. Synthesis and Ligand Exchange of Thiol-Capped Silicon Nanocrystals. Langmuir 2015, 31, 6886–6893. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhong, Y.; Jiang, X.; Peng, F.; Lu, Y.; Ji, X.; Su, Y.; He, Y. Peptide-Conjugated Fluorescent Silicon Nanoparticles Enabling Simultaneous Tracking and Specific Destruction of Cancer Cells. Anal. Chem. 2015, 87, 6718–6723. [Google Scholar] [CrossRef] [PubMed]
- Biesta, W.; van Lagen, B.; Gevaerts, V.S.; Marcelis, A.T.M.; Paulusse, J.M.J.; Nielen, M.W.F.; Zuilhof, H. Correction to Preparation, Characterization, and Surface Modification of Trifluoroethyl Ester-Terminated Silicon Nanoparticles. Chem. Mater. 2012, 24, 4782. [Google Scholar] [CrossRef]
- Heinrich, J.L.; Curtis, C.L.; Credo, G.M.; Sailor, M.J.; Kavanagh, K.L. Luminescent colloidal silicon suspensions from porous silicon. Science 1992, 255, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; Erogbogbo, F.; Swihart, M.T.; Ruckenstein, E. Organically capped silicon nanoparticles with blue photoluminescence prepared by hydrosilylation followed by oxidation. Langmuir 2006, 22, 4363–4370. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Swihart, M.T. Surface Functionalization of Silicon Nanoparticles Produced by Laser-Driven Pyrolysis of Silane followed by HF−HNO 3 Etching. Langmuir 2004, 20, 4720–4727. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Peng, F.; Bao, F.; Wang, S.; Zhong, Y.; Peng, F.; Bao, F.; Wang, S.; Ji, X.; Yang, L.; et al. Large-Scale Aqueous Synthesis of Fluorescent and Biocompatible Silicon Nanoparticles and Their Use as Highly Photostable Biological Probes Large-Scale Aqueous Synthesis of Fluorescent and Biocompatible Silicon Nanoparticles and Their Use as Highly Photost. J. Am. Chem. Soc. 2013, 135, 8350–8356. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Sun, X.; Wang, S.; Peng, F.; Bao, F.; Su, Y.; Li, Y.; Lee, S.-T.; He, Y. Facile, Large-Quantity Synthesis of Stable, Tunable-Color Silicon Nanoparticles and Their Application for Long-Term Cellular Imaging. ACS Nano 2015, 9, 5958–5967. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Chen, Y.; Feng, J.; Liu, J.; Zuo, X.; Chen, X. One-Step Synthesis of Water-Dispersible and Biocompatible Silicon Nanoparticles for Selective Heparin Sensing and Cell Imaging. Anal. Chem. 2016, 88, 10474–10481. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Cauda, V.; Argyo, C.; Bein, T. Impact of different PEGylation patterns on the long-term bio-stability of colloidal mesoporous silica nanoparticles. J. Mater. Chem. 2010, 20, 8693. [Google Scholar] [CrossRef]
- Oliveira, E.; Santos, H.M.; Jorge, S.; Rodríguez-González, B.; Novio, F.; Lorenzo, J.; Ruiz-Molina, D.; Capelo, J.L.; Lodeiro, C. Sustainable Synthesis of Luminescent CdTe Quantum Dots coated with Modified Silica Mesoporous Nanoparticles: Towards new proteins scavengers and smart drug delivery carriers. Dyes Pigment. 2018. submitted. [Google Scholar]
- Wu, S.; Dou, J.; Zhang, J.; Zhang, S. A simple and economical one-pot method to synthesize high-quality water soluble CdTe QDs. J. Mater. Chem. 2012, 22, 14573. [Google Scholar] [CrossRef]
- Bouchoucha, M.; Côté, M.F.; C-Gaudreault, R.; Fortin, M.A.; Kleitz, F. Size-Controlled Functionalized Mesoporous Silica Nanoparticles for Tunable Drug Release and Enhanced Anti-Tumoral Activity. Chem. Mater. 2016, 28, 4243–4258. [Google Scholar] [CrossRef]
- Martins, M.J.; Roque Bravo, R.; Enea, M.; Carmo, H.; Carvalho, F.; Bastos, M.L.; Dinis-Oliveira, R.J.; Dias da Silva, D. Ethanol addictively enhances the in vitro cardiotoxicity of cocaine through oxidative damage, energetic deregulation, and apoptosis. Arch. Toxicol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Dias da Silva, D.; Carmo, H.; Lynch, A.; Silva, E. An insight into the hepatocellular death induced by amphetamines, individually and in combination: The involvement of necrosis and apoptosis. Arch. Toxicol. 2013, 87, 2165–2185. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Boyle, A.L.; Friedrich, H.; Bomans, P.H.H.; Bussmann, J.; Sommerdijk, N.A.J.M.; Jiskoot, W.; Kros, A. Mesoporous Silica Nanoparticles with Large Pores for the Encapsulation and Release of Proteins. ACS Appl. Mater. Interfaces 2016, 8, 32211–32219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rida, M.A.; Harb, F. Synthesis and Characterization of Amorphous Silica Nanoparitcles from Aqueous Silicates Uisng Cationic Surfactants. J. Met. Mater. Miner. 2014, 24, 37–42. [Google Scholar] [CrossRef]
- Lin, I.-C.; Liang, M.; Liu, T.-Y.; Jia, Z.; Monteiro, M.J.; Toth, I. Effect of polymer grafting density on silica nanoparticle toxicity. Bioorg. Med. Chem. 2012, 20, 6862–6869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Bai, X.; Jiang, T.; Zhang, Q.; Wang, S. Mesoporous Silica Nanoparticles for Increasing the Oral Bioavailability and Permeation of Poorly Water Soluble Drugs. Mol. Pharm. 2012, 9, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Pisani, C.; Rascol, E.; Dorandeu, C.; Charnay, C.; Guari, Y.; Chopineau, J.; Devoisselle, J.-M.; Prat, O. Biocompatibility assessment of functionalized magnetic mesoporous silica nanoparticles in human HepaRG cells. Nanotoxicology 2017, 11, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.C.; Rippstein, P.; Tayabali, A.F.; Willmore, W.G. Mitochondrial Toxicity of Cadmium Telluride Quantum Dot Nanoparticles in Mammalian Hepatocytes. Toxicol. Sci. 2015, 146, 31–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadaf, A.; Zeshan, B.; Wang, Z.; Zhang, R.; Xu, S.; Wang, C.; Cui, Y. Toxicity Evaluation of Hydrophilic CdTe Quantum Dots and CdTe@SiO 2 Nanoparticles in Mice. J. Nanosci. Nanotechnol. 2012, 12, 8287–8292. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, P.; Wang, Y.; Zhu, Z.; Lao, F.; Liu, X.; Cong, W.; Chen, C.; Gao, Y.; Liu, Y. The Influence on Cell Cycle and Cell Division by Various Cadmium-Containing Quantum Dots. Small 2013, 9, 2440–2451. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; He, Y.; Su, Y.; Li, X.; Huang, Q.; Wang, H.; Zhang, X.; Tai, R.; Fan, C. The cytotoxicity of cadmium-based quantum dots. Biomaterials 2012, 33, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Jing, F.; Lin, B.; Cui, S.; Yu, R.; Shen, X.; Wang, T. pH-Responsive Magnetic Mesoporous Silica-Based Nanoplatform for Synergistic Photodynamic Therapy/Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 15001–15011. [Google Scholar] [CrossRef] [PubMed]











© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcelo, G.; Ariana-Machado, J.; Enea, M.; Carmo, H.; Rodríguez-González, B.; Luis Capelo, J.; Lodeiro, C.; Oliveira, E. Toxicological Evaluation of Luminescent Silica Nanoparticles as New Drug Nanocarriers in Different Cancer Cell Lines. Materials 2018, 11, 1310. https://doi.org/10.3390/ma11081310
Marcelo G, Ariana-Machado J, Enea M, Carmo H, Rodríguez-González B, Luis Capelo J, Lodeiro C, Oliveira E. Toxicological Evaluation of Luminescent Silica Nanoparticles as New Drug Nanocarriers in Different Cancer Cell Lines. Materials. 2018; 11(8):1310. https://doi.org/10.3390/ma11081310
Chicago/Turabian StyleMarcelo, Gonçalo, Jessica Ariana-Machado, Maria Enea, Helena Carmo, Benito Rodríguez-González, José Luis Capelo, Carlos Lodeiro, and Elisabete Oliveira. 2018. "Toxicological Evaluation of Luminescent Silica Nanoparticles as New Drug Nanocarriers in Different Cancer Cell Lines" Materials 11, no. 8: 1310. https://doi.org/10.3390/ma11081310