Evaluation of Bone Sialoprotein Coating of Three-Dimensional Printed Calcium Phosphate Scaffolds in a Calvarial Defect Model in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scaffold Preparation and Coating
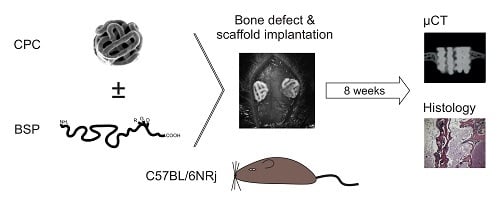
2.2. Study Design
2.3. Anaesthesia and Surgical Procedure
2.4. Micro Computed Tomography (µCT) Analyses
2.5. Measurements of Bone Thickness at the Bone–Implant Interface
2.6. Bone Volume/Total Volume Ratio
2.7. Histological Analysis
2.8. Immunohistological Analysis
2.9. Statistical Analyses
3. Results
3.1. Qualitative Evaluation of BSP Coating
3.2. Bone Thickness
3.3. Bone Volume/Total Volume (BV/TV)
3.4. Histology
3.4.1. HE Stainings
3.4.2. Masson-Goldner-Trichrom (MGT) Staining
3.4.3. Immunohistological Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dumic-Cule, I.; Pecina, M.; Jelic, M.; Jankolija, M.; Popek, I.; Grgurevic, L.; Vukicevic, S. Biological aspects of segmental bone defects management. Int. Orthop. 2015, 39, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Abudu, A.; Grimer, R.J.; Carter, S.R.; Tillman, R.M. The treatment of benign lesions of the proximal femur with non-vascularised autologous fibular strut grafts. J. Bone Jt. Surg. Br. Vol. 2008, 90, 648–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, G.; Narayan, B. Morbidity at bone graft donor sites. In Classic Papers in Orthopaedics; Springer: Berlin, Germany, 2014; pp. 503–505. ISBN 9781447154518. [Google Scholar]
- Ebraheim, N.A.; Elgafy, H.; Xu, R. Bone-graft harvesting from iliac and fibular donor sites: techniques and complications. J. Am. Acad. Orthop. Surg. 2001, 9, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.-M.M. Review: Calcium Phosphate Cements (CPCs) for bone substitution: chemistry, handling and mechanical properties. Acta Biomater. 2013, 10, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Alessandro, R.; Brucato, V.; Rimondini, L.; Spadaro, G. I materiali biocompatibili per la medicina/Biomaterials for Medicine; Universitas Studiorum: Mantua, Italy, 2014; ISBN 9788897683520. [Google Scholar]
- Lode, A.; Meissner, K.; Luo, Y.; Sonntag, F.; Glorius, S.; Nies, B.; Vater, C.; Despang, F.; Hanke, T.; Gelinsky, M. Fabrication of porous scaffolds by three-dimensional plotting of a pasty calcium phosphate bone cement under mild conditions. J. Tissue Eng. Regen. Med. 2014, 8, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, 3–6. [Google Scholar] [CrossRef]
- Rapuano, B.E.; Wu, C.; MacDonald, D.E. Osteoblast-like cell adhesion to bone sialoprotein peptides. J. Orthop. Res. 2004, 22, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Gordon, J.A.R.; Tye, C.E.; Sampaio, A.V.; Underhill, T.M.; Hunter, G.K.; Goldberg, H.A. Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone 2007, 41, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, A.; Klein, A.; Ritz, U.; Ackermann, A.; Anthonissen, J.; Kaufmann, K.B.; Brendel, C.; Götz, H.; Rommens, P.M.; Hofmann, A. Surface functionalization of orthopedic titanium implants with bone sialoprotein. PLoS ONE 2016, 11, e0153978. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Baranowski, A.; Ritz, U.; Götz, H.; Heinemann, S.; Mattyasovszky, S.; Rommens, P.M.; Hofmann, A. Effect of bone sialoprotein coated three-dimensional printed calcium phosphate scaffolds on primary human osteoblasts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.C.; Salih, E.; Gallagher, C.; FitzPatrick, D.; O’Higgins, N.; O’Rourke, S.K. Bone sialoprotein-coated femoral implants are osteoinductive but mechanically compromised. J. Orthop. Res. 2004, 22, 641–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Kennedy, J.G.; Kasser, J.R.; Glimcher, M.J.; Salih, E. Novel bioactive property of purified native bone sialoprotein in bone repair of a calvarial defect. Trans.Orthop. Res. Soc. 1998, 23, 1007. (In Japanese) [Google Scholar]
- Wang, J.; Maniwa, S.G.M. Temporal and spatial independence of bone and cartilage induction by demineralized bone powder in cranial defects of rats. J. Bone Mni. Res. 1994, 9, 181. [Google Scholar]
- Malaval, L.; Monfoulet, L.; Fabre, T.; Pothuaud, L.; Bareille, R.; Miraux, S.; Thiaudiere, E.; Raffard, G.; Franconi, J.M.; Lafage-Proust, M.H.; et al. Absence of bone sialoprotein (BSP) impairs cortical defect repair in mouse long bone. Bone 2009, 45, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Wade-Gueye, N.M.; Boudiffa, M.; Vanden-Bossche, A.; Laroche, N.; Aubin, J.E.; Vico, L.; Lafage-Proust, M.H.; Malaval, L. Absence of bone sialoprotein (BSP) impairs primary bone formation and resorption: The marrow ablation model under PTH challenge. Bone 2012, 50, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Bellahcene, A.; Bonjean, K.; Fohr, B.; Fedarko, N.S.; Robey, F.A.; Young, M.F.; Fisher, L.W.; Castronovo, V. Bone Sialoprotein Mediates Human Endothelial Cell Attachment and Migration and Promotes Angiogenesis. Circ. Res. 2000, 86, 885–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tye, C.E.; Rattray, K.R.; Warner, K.J.; Gordon, J.A.R.; Sodek, J.; Hunter, G.K.; Goldberg, H.A. Delineation of the hydroxyapatite-nucleating domains of bone sialoprotein. J. Biol. Chem. 2003, 278, 7949–7955. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.K.; Goldberg, H.A. Nucleation of hydroxyapatite by bone sialoprotein. Proc. Natl. Acad. Sci. USA 1993, 90, 8562–8565. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Rössler, S.; Lemm, M.; Ruhnow, M.; Nies, B. Properties of injectable ready-to-use calcium phosphate cement based on water-immiscible liquid. Acta Biomater. 2013, 9, 6199–6207. [Google Scholar] [CrossRef] [PubMed]
- Hertweck, J.; Ritz, U.; Götz, H.; Schottel, P.C.; Rommens, P.M.; Hofmann, A. CD34+ cells seeded in collagen scaffolds promote bone formation in a mouse calvarial defect model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doube, M.; Klosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mark, M.P.; Butler, W.T.; Prince, C.W.; Finkelman, R.D.; Ruch, J.V. Developmental expression of 44-kDa bone phosphoprotein (osteopontin) and bone γ-carboxyglutamic acid (Gla)-containing protein (osteocalcin) in calcifying tissues of rat. Differentiation 1988, 37, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of Biomaterial–Cell Interactions by Adsorbed Proteins: A Review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef] [PubMed]
- Mackie, E.J.; Ramsey, S. Modulation of osteoblast behaviour by tenascin. J. Cell Sci. 1996, 109, 1597–1604. [Google Scholar] [PubMed]
- D’Aquino, R.; Graziano, A.; Sampaolesi, M.; Laino, G.; Pirozzi, G.; De Rosa, A.; Papaccio, G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: A pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007, 14, 1162–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernike, E.; Hofstetter, W.; Liu, Y.; Wu, G.; Sebald, H.J.; Wismeijer, D.; Hunziker, E.B.; Siebenrock, K.A.; Klenke, F.M. Long-term cell-mediated protein release from calcium phosphate ceramics. J. Biomed. Mater. Res. Part A 2010, 92, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Akkineni, A.R.; Luo, Y.; Schumacher, M.; Nies, B.; Lode, A.; Gelinsky, M. 3D plotting of growth factor loaded calcium phosphate cement scaffolds. Acta Biomater. 2015, 27, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Graf, H.L.; Stoeva, S.; Armbruster, F.P.; Neuhaus, J.; Hilbig, H. Effect of bone sialoprotein and collagen coating on cell attachment to TICER® and pure titanium implant surfaces. Int. J. Oral Maxillofac. Surg. 2008, 37, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Hilbig, H.; Kirsten, M.; Rupietta, R.; Graf, H.L.; Thalhammer, S.; Strasser, S.; Armbruster, F.P. Implant surface coatings with bone sialoprotein, collagen, and fibronectin and their effects on cells derived from human maxillar bone. Eur. J. Med. Res. 2007, 12, 6–12. [Google Scholar] [PubMed]
- Schaeren, S.; Jaquiéry, C.; Wolf, F.; Papadimitropoulos, A.; Barbero, A.; Schultz-Thater, E.; Heberer, M.; Martin, I. Effect of bone sialoprotein coating of ceramic and synthetic polymer materials on in vitro osteogenic cell differentiation and in vivo bone formation. J. Biomed. Mater. Res. Part A 2010, 92, 1461–1467. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, J.Y.; Chung, C.P.; Park, Y.J. Enhanced osteogenesis by collagen-binding peptide from bone sialoprotein in vitro and in vivo. J. Biomed. Mater. Res. Part A 2013, 101, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.D.; Goldberg, H.A.; Hunter, G.K.; Dixon, S.J.; Rizkalla, A.S. Modification of polymer networks with bone sialoprotein promotes cell attachment and spreading. J. Biomed. Mater. Res. Part A 2010, 94, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Kruger, T.E.; Miller, A.H.; Wang, J. Collagen scaffolds in bone sialoprotein-mediated bone regeneration. Sci. World J. 2013, 2013, 812718. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Anderson, A.L.; Lu, Q.; Wang, J. Role of fibrillar structure of collagenous carrier in bone sialoprotein-mediated matrix mineralization and osteoblast differentiation. Biomaterials 2007, 28, 750–761. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranowski, A.; Klein, A.; Ritz, U.; Götz, H.; Mattyasovszky, S.G.; Rommens, P.M.; Hofmann, A. Evaluation of Bone Sialoprotein Coating of Three-Dimensional Printed Calcium Phosphate Scaffolds in a Calvarial Defect Model in Mice. Materials 2018, 11, 2336. https://doi.org/10.3390/ma11112336
Baranowski A, Klein A, Ritz U, Götz H, Mattyasovszky SG, Rommens PM, Hofmann A. Evaluation of Bone Sialoprotein Coating of Three-Dimensional Printed Calcium Phosphate Scaffolds in a Calvarial Defect Model in Mice. Materials. 2018; 11(11):2336. https://doi.org/10.3390/ma11112336
Chicago/Turabian StyleBaranowski, Andreas, Anja Klein, Ulrike Ritz, Hermann Götz, Stefan G. Mattyasovszky, Pol M. Rommens, and Alexander Hofmann. 2018. "Evaluation of Bone Sialoprotein Coating of Three-Dimensional Printed Calcium Phosphate Scaffolds in a Calvarial Defect Model in Mice" Materials 11, no. 11: 2336. https://doi.org/10.3390/ma11112336