Ordering of Primary Carbonitrides in an Austenitic Steel Revealed by Transmission Electron Microscopy and Atom Probe Tomography
Abstract
:1. Introduction
2. Experimental Procedures
3. Results
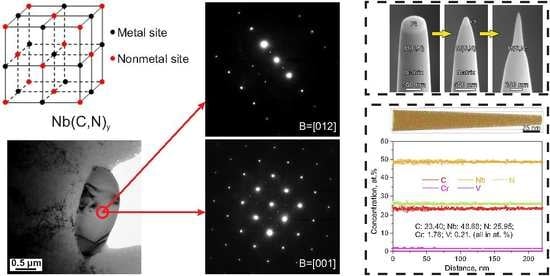
3.1. Carbonitrides in Solution-Treated Steel
3.2. Carbonitrides in Long-Term Aged Steel
4. Discussion
4.1. Carbon Diffusion
4.2. Ordering of the Primary Carbonitrides
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sawaragi, Y.; Hirano, S. New Alloys for Pressure Vessels and Piping; ASME: New York, NY, USA, 1990; pp. 141–146. [Google Scholar]
- Sawaragi, Y.; Otsuka, N.; Senba, H.; Yamamoto, S. Properties of a new 18-8 austenitic steel tube (Super 304H) for fossil fired boilers after service exposure with high elevated temperature strength. Sumitomo Search 1994, 56, 34–43. [Google Scholar]
- Viswanathan, R.; Henry, J.F.; Tanzosh, J.; Stanko, G.; Shingledecker, J.; Vitalis, B.; Purgert, R. US program on materials technology for ultra-supercritical coal power plants. J. Mater. Eng. Perform. 2005, 14, 281–292. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H. Recent developments in stainless steels. Mater. Sci. Eng. R 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Gusev, A.I.; Rempel, A.A. Phase diagrams of metal–carbon and metal–nitrogen systems and ordering in strongly nonstoichiometric carbides and nitrides. Phys. Status Solidi (a) 1997, 163, 273–304. [Google Scholar] [CrossRef]
- Gusev, A.I.; Rempel, A.A. Atomic ordering and phase equilibria in strongly nonstoichiometric carbides and nitrides. In Materials Science of Carbides, Nitrides and Borides; Gogotsi, Y.G., Andrievski, R.A., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 47–64. [Google Scholar]
- Gusev, A.I.; Rempel, A.A.; Magerl, A.J. Disorder and Order in Strongly Nonstoichiometric Compounds: Transition Metal Carbides, Nitrides and Oxides, 1st ed.; Springer: New York, NY, USA, 2001. [Google Scholar]
- Gusev, A.I. Order–disorder transformations and phase equilibria in strongly nonstoichiometric compounds. Phys. Uspekhi 2000, 43, 1–37. [Google Scholar] [CrossRef]
- Gusev, A.I. Nonstoichiometry, Disorder, Short-Range and Long-Range Orders in a Solid State; Fizmatlit: Moscow, Russia, 2007. [Google Scholar]
- Gusev, A.I.; Rempel, A.A. Nonstoichiometry, Disorder and Order in Solids; Ural Division of the Russian Academy of Sciences: Ekaterinburg, Russia, 2001. [Google Scholar]
- Gusev, A.I. Disorder and long-range order in non-stoichiometric interstitial compounds transition metal carbides, nitrides, and oxides. Phys. Status Solidi (b) 1991, 163, 17–54. [Google Scholar] [CrossRef]
- Venables, J.D.; Kahn, D.; Lye, R.G. Structure of ordered compound V6C5. Philos. Mag. 1968, 18, 177–192. [Google Scholar] [CrossRef]
- Billingham, J.; Bell, P.S.; Lewis, M.H. Vacancy short-range order in substoichiometric transition metal carbides and nitrides with the NaCl structure. I. Electron diffraction studies of short-range ordered compounds. Acta Crystallogr. Sect. A 1972, 28, 602–606. [Google Scholar] [CrossRef] [Green Version]
- Landesman, J.P.; Christensen, A.N.; de Novion, C.H.; Lorenzelli, N.; Convert, P. Order-disorder transition and structure of the ordered vacancy compound Nb6C5: powder neutron diffraction studies. J. Phys. C Solid State Phys. 1985, 18, 809–823. [Google Scholar] [CrossRef]
- Christensen, A.N. Vacancy order in Nb6C5. Acta Chem. Scand. Ser. A 1985, 39, 803–804. [Google Scholar] [CrossRef]
- Nowotny, J.; Weppner, W. Non-Stoichiometric Compounds: Surfaces, Grain Boundaries and Structural Defects; Springer: Dordrecht, The Netherlands, 1989. [Google Scholar]
- Arbuzov, M.P.; Khaenko, B.V.; Sivak, O.P. Superstructures of order in niobium monocarbide. Doklady Akademii Nauk Ukrainskoj SSR Serija A 1984, 10, 86–88. [Google Scholar]
- Khaenko, B.V.; Sivak, O.P.; Sinel’nikova, V.S. X-ray study of ordered modification of niobium monocarbide. Izv. Akad. Nauk SSSR Neorg. Mater. 1984, 20, 1825–1828. [Google Scholar]
- Khaenko, B.V.; Sivak, O.P. Order structure of niobium monocarbide. Sov. Phys. Crystallogr. 1990, 35, 653–655. [Google Scholar]
- Li, Z.; Tasan, C.C.; Pradeep, K.G.; Raabe, D. A TRIP-assisted dual-phase high-entropy alloy: Grain size and phase fraction effects on deformation behavior. Acta Mater. 2017, 131, 323–335. [Google Scholar] [CrossRef]
- Toth, L.E. Transition Metal Carbides and Nitrides; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Chi, C.Y.; Yu, H.Y.; Dong, J.X.; Liu, W.Q.; Cheng, S.C.; Liu, Z.D.; Xie, X.S. The precipitation strengthening behavior of Cu-rich phase in Nb contained advanced Fe–Cr–Ni type austenitic heat resistant steel for USC power plant application. Prog. Nat. Sci. Mater. Int. 2012, 22, 175–185. [Google Scholar] [CrossRef]
- Ou, P.; Sun, J.; Cui, Z.Q.; Yang, C.S. Microstructure of Super304H austenitic heat-resistant steel after long-term aging. Trans. Mater. Heat Treat. 2014, 35, 85–91. [Google Scholar]
- Hillert, M.; Staffansson, L.I. The regular-solution model for stoichiometric phases and ionic melts. Acta Chem. Scand. 1970, 24, 3618–3626. [Google Scholar] [CrossRef]
- Hillert, M. The compound energy formalism. J. Alloys Compd. 2001, 320, 161–176. [Google Scholar] [CrossRef]
- Inoue, K.; Ishikawa, N.; Ohnuma, I.; Ohtani, H.; Ishida, K. Calculation of phase equilibria between austenite and (Nb,Ti,V)(C,N) in microalloyed steels. ISIJ Int. 2001, 41, 175–182. [Google Scholar] [CrossRef]
- Ohtani, H.; Hasebe, M.; Nishizawa, T. Calculation of the Fe–C–Nb ternary phase diagram. Calphad 1989, 13, 183–204. [Google Scholar] [CrossRef]
- Hillert, M.; Nilsson, K.; Törndahl, L.E. Effect of alloying elements on the formation of austenite and dissolution of cementite. J. Iron Steel Inst. 1971, 209, 49–66. [Google Scholar]
- Lipatnikov, V.N.; Gusev, A.I. Crystal structure and microstructure of disordered and ordered vanadium carbonitrides. Inorg. Mater. 2007, 43, 827–833. [Google Scholar] [CrossRef]
- Gusev, A.I.; Lipatnikov, V.N. Effect of ordering on the structure and heat capacity of cubic vanadium carbonitrides VCxNy. JETP Lett. 2007, 84, 598–603. [Google Scholar] [CrossRef]
- Gusev, A.I.; Rempel, A.A. Superstructures of non-stoichiometric interstitial compounds and the distribution functions of interstitial atoms. Phys. Status Solidi (a) 1993, 135, 15–58. [Google Scholar] [CrossRef]
- Gusev, A.I. Nonstoichiometry and superstructures. Phys. Uspekhi 2014, 57, 839–876. [Google Scholar] [CrossRef]
- Faizullaev, F.; Karimov, I.; Kalanov, M.; Emiraliev, A. Cubic ordering in vanadium carbonitrides. Izv. Akad. Nauk SSSR Ser. Fiz.-Mat. Nauk 1979, 5, 74–76. [Google Scholar]








| C | Mn | P | S | Si | Ni | Cr | Cu | Nb | N | Al | B | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.08 | 0.787 | 0.022 | 0.001 | 0.29 | 8.88 | 17.98 | 3.066 | 0.58 | 0.07 | 0.012 | 0.0026 | Bal. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, P.; Li, Z. Ordering of Primary Carbonitrides in an Austenitic Steel Revealed by Transmission Electron Microscopy and Atom Probe Tomography. Materials 2018, 11, 2321. https://doi.org/10.3390/ma11112321
Ou P, Li Z. Ordering of Primary Carbonitrides in an Austenitic Steel Revealed by Transmission Electron Microscopy and Atom Probe Tomography. Materials. 2018; 11(11):2321. https://doi.org/10.3390/ma11112321
Chicago/Turabian StyleOu, Ping, and Zhiming Li. 2018. "Ordering of Primary Carbonitrides in an Austenitic Steel Revealed by Transmission Electron Microscopy and Atom Probe Tomography" Materials 11, no. 11: 2321. https://doi.org/10.3390/ma11112321