Simplification of a Mechanistic Model of Biomass Combustion for On-Line Computations
Abstract
:1. Introduction
2. Process Description of a BioGrate Boiler
3. Model Development for Process Control and Monitoring
3.1. Mechanistic Model
3.1.1. Modelling of Mass Conservation in the Solid Phase
3.1.2. Modelling of Mass Conservation in the Gas Phase
3.1.3. Modelling of Energy Conservation in the Solid Phase
3.1.4. Modelling of the Gas Phase Energy Conservation
3.2. Development of a Simplified Dynamic Model
3.3. Approach for Simplification of the Mechanistic Model
3.4. Mathematical Formulation of the Simplified Model
Mathematical Formulation of the Dynamic Model for On-Line Computations
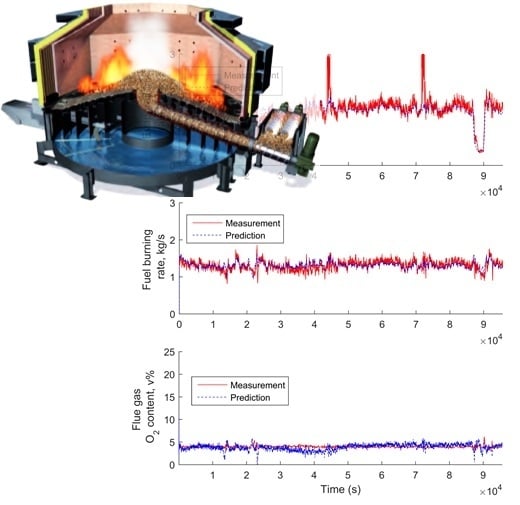
3.5. Simplified Model Validation with Industrial Data from a BioGrate Boiler
4. Performance Evaluation of the Simplified Model Predictions against the Predictions from the Mechanistic Model and Autoregressive Model with Exogenous Inputs
4.1. Comparison of the Simplified Dynamic Model against the Mechanistic Model
4.2. Comparison of the Simplified Dynamic Model against a Linear Autoregressive Model with Exogenous Inputs Model
4.3. Comparison of Computational Times of Different Models
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ∆HCO | Enthalpy of CO formation (kJ/mol) |
| ∆HCO2 | Enthalpy of CO2 formation (kJ/mol) |
| ∆Hevap | Enthalpy of vaporization (kJ/kg) |
| ∆Hp,i | Pyrolysis enthalpy of component i (kJ/kg) |
| A | Pre-exponential factor (s−1) |
| CCH4 | Concentration of methane (mol/cm3) |
| CCO | Concentration of carbon monoxide (mol/cm3) |
| CH2O | Concentration of steam (mol/cm3) |
| CO2 | Concentration of oxygen (mol/cm3) |
| Cp,O2 | Heat capacity of oxygen (J/(kg K)) |
| Cp,H2O,g | Heat capacity of water vapor (J/(kg K)) |
| Cp,N2 | Heat capacity of nitrogen (J/(kg K)) |
| Cp,c | Heat capacity of char (J/(kg∙K)) |
| Cp,G | Heat capacity of pyrolytic gas (J/(kg K)) |
| Cp,H2O | Heat capacity of liquid water (J/(kg∙K)) |
| Cp,w | Heat capacity of wood (J/(kg∙K)) |
| Cs | Heat capacity of the solid phase (J/(kg∙K)) |
| dcavity | Average cavity diameter (m) |
| Dg,i | Gas-phase diffusivity of component i in air (m2/s) |
| dp | Particle diameter (m) |
| E | Activation energy (J/mol) |
| FIn | Total volumetric air flow to the furnace (m3/s) |
| Fout | Total volumetric flow out of the furnace (m3/s) |
| h | Heat transfer coefficient (W m−2) |
| hC | Fraction of char in the total amount of material on the grate |
| Hg | Enthalpy of the gas phase (J/kg) |
| HG | Energy content of the gas phase (J) |
| hi,eff | Effective mass transfer coefficient |
| I− | Energy flux in a negative direction (W/m2) |
| I+ | Energy flux in a positive direction (W/m2) |
| ka | Absorption coefficient (m−1) |
| kair | Thermal conductivity of air |
| kbed | Effective heat conduction coefficient of the packed bed (W/(m∙K)) |
| kC | Reaction rate of char combustion reaction (1/s) |
| keff,i | Effective reaction constant of a heterogeneous reaction i (kg/(m3∙s)) |
| kfiber | Heat conductivity of wood fiber (W/(m∙K)) |
| kg | Heat conductivity of the gas (W/(m∙K)) |
| kmax | Maximum heat transfer coefficient (W/(m∙K)) |
| kmin | Minimal heat conduction coefficient (W/(m∙K)) |
| kO2 | Mass transfer coefficient of oxygen to the char particle (m/s) |
| kp,i | Rate constant for pyrolysis of component i |
| kpyr,comb | Rate constant of combustion of pyrolytic gas (1/s) |
| ks | Scattering coefficient (m−1) |
| kr,C | Rate constant for the char reaction with oxygen (kg/(m3∙s)) |
| kR,CO2 | Rate constant for the char reaction with carbon dioxide (kg/(m3∙s)) |
| ks,eff | Heat conduction coefficient of the solid matter (W/(m∙K)) |
| kr,H2O | Rate constant for the char reaction with water (kg/(m3∙s)) |
| kr,i | Reaction rate constant for the component i (kg/(m3∙s)) |
| ks,rad | Heat radiation coefficient of the solid matter (W/(m∙K)) |
| MCO | Molar mass of CO (kg/mol) |
| MCO2 | Molar mass of CO2 (kg/mol) |
| mC | Amount of char in the furnace (kg) |
| mCO | Amount of carbon monoxide in the furnace (kg) |
| mfuel | Amount of fuel in the furnace (kg) |
| mH2O,i | Amount of moisture in the furnace (kg) |
| mIn | Fuel feed to the furnace (kg/s) |
| mm | Predicted weight of component m (kg) |
| mO2 | Amount of air in the furnace (kg) |
| mp,G | Total amount of pyrolytic gas in the furnace (kg) |
| mp,i | Amount of volatile component i in the furnace (kg) |
| mH2O,l | Amount of moisture on the grate (kg) |
| mH2O | Amount of water vapor in the fuel layer (kg) |
| Pr | Prandatl number |
| Qg,i | Energy produced or consumed by a gas phase reaction i (J/(m3∙s)) |
| Qs | Energy released through radiation and convection (W) |
| Qs,i | Energy produced or consumed by a solid phase reaction i (J/(m3∙s)) |
| QC | Energy from char combustion (J/s) |
| Qin | Energy contained in primary air (J/s) |
| Qout | Energy of outflowing gas (J/s) |
| rC | Reaction rate of char (kg/s) |
| Re | Reynolds number |
| revap | Drying rate (kg/(m3∙s)) |
| revap,conv | Rate of moisture evaporation due to the heat transfer between gas and solid |
| revap,rad | Rate of moisture evaporation due to the radiative heat transfer from char layer |
| rg,CH4 | Oxidation rate of methane (kg/(m3∙s)) |
| rg,CO | Oxidation rate of the carbon monoxide (kg/(m3∙s)) |
| rg,H2 | Oxidation rate of hydrogen (kg/(m3∙s)) |
| rg,i | Reaction rate of the gaseous component i (kg/(m3∙s)) |
| rp,i | Rate of pyrolysis of component i (kg/s) |
| rpyr | Rate of pyrolysis reaction (kg/s) |
| rpyr,comb | Rate of combustion of pyrolytic gas (kg/s) |
| rs,H2O | Drying rate of fuel (kg/(m3∙s)) |
| rs,j | Rate of reaction of the solid component j (kg/(m3∙s)) |
| rs,pyr | Reaction rate of pyrolysis (kg/(m3∙s)) |
| S | Density number (m−1) |
| Sc | Schmidt number |
| Tg | Temperature of the gas phase (K) |
| TIn | Temperature of the fed air flow (K) |
| Ts | Temperature of the solid (K) |
| t | Time variable (s) |
| V | Volume of the material on the grate (m3) |
| vg | Gas flow velocity (m/s) |
| X | Degree of conversion of char |
| XC | Char fraction in the pyrolysis products |
| XH2O | Moisture content of the fuel |
| Xm | Moisture content of the fuel in the furnace |
| Xp,G | Fraction of pyrolysis gas in the wood pyrolysis |
| x | Vertical coordinate (m) |
| Yg,i | Mass fraction of the gaseous component i |
| εb | Bed porosity |
| εp | Particle porosity |
| κconv | Heat convection coefficient (W/(m2·K)) |
| κs,eff | Effective heat conduction coefficient of the solid matter (W/(m∙K)) |
| ρ | Density of the fluid (kg/m3) |
| ρAir | Density of air (kg/m3) |
| ρc | Mass concentration of char (kg/m3) |
| ρCO | Mass concentration of carbon monoxide (kg/m3) |
| ρg | Mass concentration of the gas phase (kg/m3) |
| ρH2 | Mass concentration of hydrogen (kg/m3) |
| ρm | Mass concentration of component m (kg/m3) |
| ρO2 | Mass concentration of oxygen (kg/m3) |
| ρs | Total mass concentration of the solid phase (kg/m3) |
| ρs,j | Mass concentration of the solid component j (kg/m3) |
| ρv | Mass concentration of volatiles (kg/m3) |
| ρw | Mass concentration of water (kg/m3) |
| σ | Stefan-Boltzman constant (W/(m2∙K4)) |
| Ω | Ratio of carbon monoxide to carbon dioxide |
| Ωpyr | Stoichiometric coefficient of pyrolytic gas combustion |
References
- Hogg, B.; El-Rabaie, N. Multivariable generalized predictive control of a boiler system. IEEE Trans. Energy Convers. 1991, 6, 282–288. [Google Scholar] [CrossRef]
- Bauer, R.; Gölles, M.; Brunner, T.; Dourdoumas, N.; Obernberger, I. Modelling of grate combustion in a medium scale biomass furnace for control purposes. Biomass Bioenergy 2010, 34, 417–427. [Google Scholar] [CrossRef]
- Gölles, M.; Reiter, S.; Brunner, T.; Dourdoumas, N.; Obernberger, I. Model based control of a small-scale biomass boiler. Control Eng. Pract. 2014, 22, 94–102. [Google Scholar] [CrossRef]
- Kortela, J.; Jämsä-Jounela, S. Model predictive control utilizing fuel and moisture soft-sensors for the BioPower 5 combined heat and power (CHP) plant. Appl. Energy 2014, 131, 189–200. [Google Scholar] [CrossRef]
- Flynn, M.; O’Malley, M. A drum boiler model for long term power system dynamic simulation. IEEE Trans. Power Syst. 1999, 14, 209–217. [Google Scholar] [CrossRef]
- Paces, N.; Kozek, M. Modeling of a Grate-Firing Biomass Furnace for Real-Time Application. In Proceedings of the International Symposium on Qualitative, Quatitative and Hybrid Models and Modeling Methodologies in Science and Engineering (MMMse 2011), Orlando, FL, USA, 27–30 March 2011.
- Belkhir, F.; Meiers, J.; Felgner, F.; Frey, G. A Biomass Combustion Plant Model for Optimal Control Applications—The Effect of Key Variables on Combustion Dynamics. In Proceedings of the 6th International Renewable Energy Congress (IREC), Sousse, Tunisia, 24–26 March 2015; pp. 1–6.
- Liu, X.; Kong, X.; Hou, G.; Wang, J. Modeling of a 1000 MW power plant ultra super-critical boiler system using fuzzy-neural network methods. Energy Convers. Manag. 2013, 65, 518–527. [Google Scholar] [CrossRef]
- Peng, H.; Nakano, K.; Shioya, H. Nonlinear predictive control using neural nets-based local linearization ARX model—Stability and industrial application. IEEE Trans. Control Syst. Technol. 2007, 15, 130–143. [Google Scholar] [CrossRef]
- Havlena, V.; Findejs, J. Application of model predictive control to advanced combustion control. Control Eng. Pract. 2005, 13, 671–680. [Google Scholar] [CrossRef]
- Leskens, M.; Van Kessel, L.; Van den Hof, P. MIMO closed-loop identification of an MSW incinerator. Control Eng. Pract. 2002, 10, 315–326. [Google Scholar] [CrossRef]
- Leskens, M.; Van Kessel, L.; Bosgra, O. Model predictive control as a tool for improving the process operation of MSW combustion plants. Waste Manag. 2005, 25, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Morari, M.; Lee, J.H. Model predictive control: Past, present and future. Comput. Chem. Eng. 1999, 23, 667–682. [Google Scholar] [CrossRef]
- Boriouchkine, A.; Zakharov, A.; Jämsä-Jounela, S. Dynamic modeling of combustion in a BioGrate furnace: The effect of operation parameters on biomass firing. Chem. Eng. Sci. 2012, 69, 669–678. [Google Scholar] [CrossRef]
- Boriouchkine, A.; Sharifi, V.; Swithenbank, J.; Jämsä-Jounela, S. A study on the dynamic combustion behavior of a biomass fuel bed. Fuel 2014, 135, 468–481. [Google Scholar] [CrossRef]
- Pomerantsev, V. Fundamentals of Applied Combustion Theory; Energoatomizdat: Moscow, Russia, 1986. [Google Scholar]
- Fogler, S. Elements of Chemical Reaction Engineering, 4th ed.; Pearson Educations: Upper Saddle River, NJ, USA, 2006. [Google Scholar]
- Branca, C.; Di Blasi, C. Devolatilization and combustion kinetics of wood chars. Energy Fuels 2003, 17, 1609–1615. [Google Scholar] [CrossRef]
- Senneca, O. Kinetics of pyrolysis, combustion and gasification of three biomass fuels. Fuel Process. Technol. 2007, 88, 87–97. [Google Scholar] [CrossRef]
- Matsumoto, K.; Takeno, K.; Ichinose, T.; Ogi, T.; Nakanishi, M. Gasification reaction kinetics on biomass char obtained as a by-product of gasification in an entrained-flow gasifier with steam and oxygen at 900–1000 °C. Fuel 2009, 88, 519–527. [Google Scholar] [CrossRef]
- Evans, D.D.; Emmons, H. Combustion of wood charcoal. Fire Saf. J. 1977, 1, 57–66. [Google Scholar] [CrossRef]
- Janssens, M.; Douglas, B. Wood and Wood Products. In Handbook of Building Materials for Fire Protection; Harper, C.A., Ed.; McGraw-Hill: New York, NY, USA, 2004. [Google Scholar]
- Shin, D.; Choi, S. The combustion of simulated waste particles in a fixed bed. Combust. Flame 2000, 121, 167–180. [Google Scholar] [CrossRef]
- Branca, C.; Blasi, C.D.; Elefante, R. Devolatilization and heterogeneous combustion of wood fast pyrolysis oils. Ind. Eng. Chem. Res. 2005, 44, 799–810. [Google Scholar] [CrossRef]
- Boriouchkine, A.; Sharifi, V.; Swithenbank, J.; Jämsä-Jounela, S. Experiments and modeling of fixed-bed debarking residue pyrolysis: The effect of fuel bed properties on product yields. Chem. Eng. Sci. 2015, 138, 581–591. [Google Scholar] [CrossRef]
- Garcìa-Pérez, M.; Chaala, A.; Pakdel, H.; Kretschmer, D.; Roy, C. Vacuum pyrolysis of softwood and hardwood biomass: Comparison between product yields and bio-oil properties. J. Anal. Appl. Pyrolysis 2007, 78, 104–116. [Google Scholar] [CrossRef]
- Wakao, N.; Kaguei, S.; Funazkri, T. Effect of fluid dispersion coefficients on particle-to-fluid heat transfer coefficients in packed beds: Correlation of nusselt numbers. Chem. Eng. Sci. 1979, 34, 325–336. [Google Scholar] [CrossRef]
- Cooper, J.; Hallett, W.L.H. A numerical model for packed-bed combustion of char particles. Chem. Eng. Sci. 2000, 55, 4451–4460. [Google Scholar] [CrossRef]













| Computer | Mechanistic (Real-Time Seconds/Simulated Second) | Simplified | ARX |
|---|---|---|---|
| Laptop | 14.173497 | 6.23 × 10−4 | 7.21 × 10−7 |
| Desktop-1 (R2014b) | 5.403695 | 5.34 × 10−4 | 6.477 × 10−7 |
| Desktop-1 (R2015b) | 31.443311 | 2.81 × 10−4 | 7.066 × 10−7 |
| Desktop-2 | 28.240536 | 2.11 × 10−4 | 5.927 × 10−7 |
| Computer | Matlab Version | RAM (GB) | CPU Model | Frequency (GHz) | # of Cores | # of Threads |
|---|---|---|---|---|---|---|
| Laptop | R2015a | 8 | Intel i5-3340M | 2.7 | 2 | 4 |
| Desktop-1 | R2014b | 12 | Intel i7 920 | 3.6 | 4 | 8 |
| Desktop-2 | R2015b | 8 | Intel Xeon E3-1230 | 3.2 | 4 | 8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boriouchkine, A.; Jämsä-Jounela, S.-L. Simplification of a Mechanistic Model of Biomass Combustion for On-Line Computations. Energies 2016, 9, 735. https://doi.org/10.3390/en9090735
Boriouchkine A, Jämsä-Jounela S-L. Simplification of a Mechanistic Model of Biomass Combustion for On-Line Computations. Energies. 2016; 9(9):735. https://doi.org/10.3390/en9090735
Chicago/Turabian StyleBoriouchkine, Alexandre, and Sirkka-Liisa Jämsä-Jounela. 2016. "Simplification of a Mechanistic Model of Biomass Combustion for On-Line Computations" Energies 9, no. 9: 735. https://doi.org/10.3390/en9090735