1. Introduction
Based on the waste hierarchy principle in EU legislation [
1], waste-to-energy (WtE) processes are to be used to maximally avoid landfilling of non-recyclable waste. With such processes, typically based on combustion, energy as well as material content of non-recyclable waste streams can still be recovered and valorized. In this manner, state-of-the-art WtE combustion processes contribute to lower consumption of (raw) materials and fossil fuels, whilst keeping the environment and material cycles in the circular economy toxic-free [
2,
3]. The development of WtE combustion technology has been driven by evolving societal needs and steadily tightening policies.
Figure 1 shows this overall development, centered around four major lines of development (i.e., DL1 to DL4).
WtE combustion processes originated as small furnaces open to the air, which were used in early times to thermally destroy batches of mixed waste, with the aim of sanitizing densely populated cities (i.e., DL1). With time and growing awareness of environmental, health, and climate issues, these early furnaces with highly uncontrolled conditions ultimately evolved into large-scale, fully continuous, complex industrial plants. These plants are designed, built, operated, and monitored to high industry standards in order to reach legally imposed emission limits (i.e., DL2) and contractually set targets of waste throughput and energy production [
4,
5]. In this context, many WtE combustion plants have also been coupled to energy conversion cycles and have nowadays become part of large-scale combined heat and power schemes that supply energy (as steam, heat, and/or electricity) to residential, public, and industrial consumers (i.e., DL3) [
5,
6,
7]. In addition, WtE combustion plants are currently exploited for recovery of (bulk) materials in a clean and inert state that can be further recycled and applied, e.g., ferrous and non-ferrous scrap metal for reuse in the metallurgical industry, bottom and boiler ash for reuse as granulate material and a cement substitute in civil construction, and products from flue gas treatment, e.g., gypsum for building purposes (i.e., DL4) [
8,
9]. Overall, WtE combustion processes comply with ever-tighter performance criteria in view of sustainability [
10].
Although initiated (by policies) in the order as shown, the different lines of development in
Figure 1 have largely evolved in parallel (i.e., DL2 to DL4). However, throughout the past two decades, WtE combustion plants have been optimized in particular to comply with the so-called R1 criterium [
11]. This criterium was introduced at the EU level to evolve towards significantly more energy-efficient thermal treatment of non-recyclable waste. Currently, WtE combustion plants that do not comply with the R1 criterium are no longer given the status of sustainable recovery operation according to EU legislation and are hence regarded as disposal facilities. The R1 criterium has therefore been an undeniable game changer in terms of integration, design, operation, and (process) control of WtE combustion plants [
5,
12].
However, despite significant technological development, critical issues remain that are due to the intrinsic properties of thermally treated waste and, in particular, the thermochemical behavior of inorganic compounds typically present in this waste. Inorganic compounds (in solid, liquid, and gaseous forms) trigger severe fouling and corrosion in WtE combustion furnaces and boilers. This typically leads to increased maintenance costs, reduced energy efficiency, and reduced plant availability [
13]. Furthermore, in waste furnaces, inorganic compounds are released from the waste in (suddenly) varying concentrations in the combustion gasses generated. This explains the high operational safety margins upheld in WtE combustion plants (i.e., typically 10–20% in excess) for the injection of sorbents and chemicals for flue gas treatment. This, in turn, results in high operational costs [
14,
15,
16].
This paper is intended as perspective review, dealing with various topics in WtE combustion plants that are associated with inorganic compounds typically present in thermally treated waste (i.e., Cl, S, P, and a wide range of metallic elements). These topics, although some of which are known and studied for a long time, have seldom been discussed in the literature from an integrated, multi-disciplinary perspective. Therefore, this paper does not provide an in-depth review of each of the topics discussed but aims to bring together and to interrelate insights that allow to identify specific needs for further research to obtain better control over inorganic compounds in industrial WtE combustion processes. For this purpose, industrial experiences are also considered—next to scientifically established facts—which are typically not (well) documented in the literature but deemed relevant for future developments.
2. Fate of Inorganic Compounds in WtE Combustion Processes
In this section, the occurrence, behavior, and fate of inorganic compounds in industrial WtE combustion processes are described in relation to different aspects, i.e., chemical and material aspects (
Section 2.1), process and engineering aspects (
Section 2.2), operational aspects (
Section 2.3), process intelligence aspects (
Section 2.4), and plant-economical aspects (
Section 2.5). The aspects and respective topics discussed are schematically shown in
Figure 2.
2.1. Chemical and Material Aspects
2.1.1. Typical Waste Sources of Inorganic Compounds
Inorganic compounds—typically comprising metallic elements—are present in numerous manmade materials and provide (newly produced) materials with a wide range of intended properties, such as strength, color fastness, and resistance to UV, O2, corrosion, heat, fire, and microbial contamination, to name only a few. Other examples include Cr-based tanning agents in leather, Sb-based flame retardants in textiles and plastics of electronic devices, Zn-based UV-protectors in packaging materials and skin care products, and Zn- and Mg-based vulcanization additives in rubbers. Examples of more recent developments include, amongst others, metal-based nanoparticle (NP) additives (e.g., Cu-NPs in fibers for antimicrobial medical textiles), nano-engineered metallic polymers (e.g., Pt and Ru in synthetic conductors, and Ag in antimicrobial packaging materials), and metal-based crystals that render functional materials with electronic, magnetic, optical, or piezoelectric characteristics (e.g., Ag and rare earth elements in solar foil applications).
Inorganic compounds also occur in contaminated substances of biological origin. Examples include chicken manure and sewage sludge, which are rich in phosphorous but also in heavy metals that prohibit their direct application as fertilizers. Additionally relevant in this regard are pruning wood from vineyards (Cu), contaminated biomass from phytoremediation (Zn, Pb, Cd, Mn, etc.), and construction wood treated with preservation agents that contain Cu, Cr, and As (“CCA”).
2.1.2. Thermochemical Behavior of Inorganic Compounds during Combustion
When exposed to high-temperature environments in furnaces of waste combustion plants, inorganic compounds exhibit so-called partitioning behavior. This implies that the constituting elements of the considered compounds are separated from each other in some way during combustion and reappear in (identical or newly formed) compounds that are distributed over: (i) fouling deposits in furnaces, boilers, and flue gas treatment units; (ii) solids/liquids that leave the combustion plants either as boiler ash, bottom ash, solid residues, and/or fluids from flue gas treatment; and (iii) gaseous/liquid/solid products that are carried further by syn-/flue gasses as acid pollutants, fly ash, and vapors. Furthermore, metallic elements (e.g., Zn, Cu, Ti, Cr, and Pb) can recombine during thermal treatment with Cl, S, P, Si, and/or O, by which they can change their chemical speciation [
17,
18].
Early knowledge on the behavior of inorganic compounds in waste, particularly in waste incinerators, was established through chemical-thermodynamic modeling of the interactions between metals, Cl, and S [
19,
20,
21]. This approach helped mainly to unravel catalytic reaction pathways of dioxins, PCBs, inorganic gaseous pollutants, and dust particles [
22]. Then, throughout the past three decades, numerous lab-scale studies have determined the typical compositions and properties of waste materials via combustion in furnaces with subsequent chemical analysis of ash (i.e., XRF/XRD or destruction and ICP-MS) [
23]. Furthermore, ash and fouling deposits sampled from boilers in full-scale plants were extensively analyzed and interpreted. For example, the melting of successive layers in these samples and the associated vaporization of inorganic compounds were measured using TGA methods in order to reconstruct the original time- and temperature-dependent formation of the samples analyzed [
24]. Efforts were also made to measure and model the (apparent) kinetics of metal partitioning [
25].
Regarding the experimental methods and setups used, lab-scale experiments on waste and (simulated) deposits are commonly executed in muffle-type furnaces with samples placed on/in recipients [
26]. Alternatively, tubular furnaces are used with air flowing laterally to the inserted waste samples [
27]. Hence, samples of waste in lab-scale studies are mostly exposed unequally to high-temperature environments of furnaces. Furthermore, the applied conditions of flow and mass and heat transfer are, in general, not (fully) characterized in these types of experiments. On the other hand, experimental data collected from flow-through reactor setups are scarce, whilst being more representative of industrial waste processes [
28]. These observations are explained by the fact that, so far, studies of inorganics have not typically been aimed at understanding physical influences (particular for thermal waste processes) on the chemical phenomena studied. Some researchers adopted a more engineering-oriented approach, but they did not target inorganic compounds in the waste [
29].
On the pilot scale, early research on waste was executed extensively in the TAMARA plant [
30]. This pilot plant was a downsized geometrical copy of an industrial grate furnace, with fairly good reproduction of typical flue gas flow conditions in full-scale plants, and was used mainly to gain an in-depth understanding of chlorine-induced corrosion issues in waste-fired steam boilers. Hence, the pilot plant did not target the dedicated study of the solid waste bed, in particular. Other setups, e.g., vertical column reactors with flow-through configuration, were better suited for studying waste particles in packed beds subjected to high temperature gradients [
31]. In this way, engineering models covering aspects of flow, mass and heat transfer, and also the kinetics of drying and ignition were compiled. (The aspect of engineering modeling is further discussed in
Section 2.4.2.) However, the focus in these studies was typically on the carbonaceous part of the waste, whilst the thermochemistry of inorganic compounds (i.e., Cl, S, P, and metallic elements) was not targeted.
Finally, experiments on full-scale waste incinerators have proven to be neither simple nor reproducible [
32,
33]. Since the residence time of waste particles on a grate of industrial size (i.e., 0.5 to 2.5 h) is high compared to the (short) residence time of the gasses released from these particles, it is hard to correlate compositions of waste solids and gasses at different positions along the grate. Literature data in this regard are fragmented and inconclusive.
2.1.3. Fouling and Corrosion in WtE Furnaces and Boilers
Industrial practice in WtE combustion plants shows that varying conditions of combustion give rise to diverging fouling patterns in boilers and variable compositions of flue gasses and boiler ash. Fouling deposits on boiler walls and tubes, when not kept under control, can cause a type of high-temperature corrosion that is very specific for waste boilers. This corrosion affects heat exchanging surfaces at contact temperatures above 420 °C (i.e., surfaces of superheater tubes) and is induced by chlorine gas (Cl
2). Chlorine gas is formed by chemical conversion of gaseous hydrogen chloride (HCl) in micropores with O
2-restricted conditions inside fouling deposits that contain Na/K-chlorides. Furthermore, when the treated waste contains significant amounts of Zn and Pb, Zn/Pb-chlorides are typically formed that can attack also heat exchanging surfaces at lower contact temperatures (i.e., surfaces of evaporator walls). The latter type of corrosion involves the formation of aggressive eutectic melts that can rapidly deteriorate large areas of boiler walls. Details on corrosion mechanisms can be found in ref. [
34,
35,
36,
37].
In order to mitigate high-temperature corrosion caused by chlorine, maintaining sufficiently high concentrations of SO
2 in the combustion gas (relative to HCl) is key in WtE plant operation [
38]. In presence of excess O
2 in the flue gas (typically up to 8–9%v) Na/K-chlorides in fouling deposits are converted to stable sulphates (i.e., K
2SO
4/Na
2SO
4) that prevent Cl
2 from being formed in micropores in the first place. Conversely, too high SO
2 concentrations enable the formation of fouling deposits rich in Na/K-polysulfates that are also associated with corrosion on evaporator membrane walls and excessive fouling in economizer bundles [
13]. As an industrial rule of thumb, the HCl/SO
2 ratio in the flue gas should be kept preferentially in a range between 1.5 and 2.5 at all times. Furthermore, an O
2 level of at least 4%v at the boiler outlet is typically recommended to promote sulphate formation from Na/K-chlorides and to discourage Cl
2 formation in fouling deposits.
2.2. Process and Engineering Aspects
2.2.1. Mode of Contact between Waste Particles and (Primary) Combustion Air
Existing industrial WtE combustion furnaces encase solid-gas environments that are highly reactive and are operated either in cross-flow mode (i.e., moving grate furnaces) or counter-flow mode (i.e., fluidized bed furnaces). The total air flow required to perform the combustion of the waste is typically divided into primary, secondary, and possibly, tertiary combustion air flows. The primary combustion air flow is supplied to the furnace from below and flows upward, through the waste bed, into the freeboard zone (i.e., the free space above the waste). The secondary combustion air flow is injected into the freeboard directly, with the aim of establishing (full or partial) oxidation of gaseous intermediate products of thermal decomposition that are carried upward by the primary combustion air. Mainly, the primary and secondary combustion air flows, together with gasses generated by combustion of the waste, make up the total flow of combustion gas that continues its way through a boiler and flue gas treatment units installed downstream in the WtE combustion plant [
5].
In moving grate furnaces, waste is pushed forward discontinuously in a stacked layer over the surface of a grate. Moving grate furnaces are proven most reliable for thermal treatment of bulky waste types, such as municipal solid waste (MSW), with LHV typically between 5.5 and 10.5 MJ/kg. Fluidized bed furnaces, on the other hand, feature an intense combustion with turbulent mixing of waste particles, primary combustion air, and possibly sand in a fiercely bubbling bed. These furnaces are typically used for thermal treatment of pre-treated and/or pre-sorted wastes, such as commercial and industrial (C&I) waste and refused derived fuels (RDF), with LHVs ranging up to 30 MJ/kg [
39,
40]. However, due to their flexibility and robustness in operation, moving grate furnaces are also often used for the treatment of high-LHV waste. Likewise, fluidized bed furnaces are convenient for the treatment of low-LHV waste, e.g., sludge from municipal wastewater treatment, under the condition of waste pre-drying and/or primary combustion air pre-heating. Overall, approximately 90% of WtE combustion plants worldwide are equipped with moving grate furnaces, whilst the remaining use fluidized bed furnaces [
39].
Depending on the furnace type applied, primary combustion air flow has multiple different functions: (i) to establish intense mechanical agitation and thermal homogenization of the waste bed, in the case of fluidized bed furnaces; (ii) to establish protection of the mechanical grate system (i.e., by convective cooling) against high-temperature impact, in the case of moving-grate furnaces; (iii) to supply O
2 to (primary) combustion reactions in the waste bed; and (iv) to carry thermochemical reaction products of primary combustion towards and into the secondary and/or post-combustion zones. The latter two functions may impact the formation and/or release of inorganic compounds in the waste bed and are therefore further discussed in
Section 2.2.2 and
Section 2.2.3.
2.2.2. Combustion Air: Oxidation Capacity of the Furnace Environment
The total flow of combustion air supplied determines the oxidation capacity of the furnace environment (i.e., the combination of the waste bed and the freeboard environment, including the entire secondary combustion zone), which can be expressed in terms of the applied air-to-fuel ratio (λ)[
41]. Combustion processes have been classified on the basis of this ratio for a long time, with λ = 1 as a main reference value, which corresponds to an overall supply of O
2 that exactly fulfills the stoichiometric need for complete oxidation of all carbon present in a defined fuel. Applied to waste combustion, a value of λ < 1 (but still >> 0) classifies a given process as waste gasification, whilst λ > 1 results in classification as waste incineration. Both gasification and incineration processes primarily aim to convert waste into a useful source of energy, i.e., an energy carrier such as syngas (in case of gasification) or recoverable heat (in case of incineration).
A third category of thermal waste conversion processes comprises waste pyrolizers, which are characterized by λ ≈ 0. Processes in this category typically target the conversion of waste to (mainly) liquid and solid products. However, as these processes in principle do not involve O2 and are strongly energy consuming, by chemical definition they strictly cannot be regarded as combustion processes. Waste pyrolizers are therefore not further considered in this paper.
Waste Incineration
To date, incineration is by far the most applied type of thermal conversion for non-recyclable waste fuels (i.e., municipal solid waste, industrial and/or commercial waste, refuse derived fuels, solid recovered fuels, sewage sludge, or similar by EU legislation) [
42]. Waste incineration processes rely on significant over-stoichiometric supply of air (i.e., 1.2 < λ < 1.5), which results in (quasi-) complete oxidation of solids and gasses generated.
Three main advantages essential to the success of waste incineration processes have meanwhile been demonstrated on the industrial scale by numerous state-of-the-art WtE plants, i.e., process auto-thermality, flexibility with regard to the waste input, and process robustness and reliability. Incineration processes also show the highest energy efficiency of all thermal conversion processes that have been proven for waste treatment on the industrial scale [
5]. However, in full-oxidizing conditions that prevail at temperatures typically ranging from 800 to 1300 °C, carbonaceous compounds in the waste thermally decompose to the smallest single-molecule products possible (i.e., CO, CO
2, NO
x, N
2O, SO
2/SO
3, HCl, H
2O, etc.). Additionally, inorganic compounds that contain metallic elements are prone to the effects of oxidation and bottom ash agglomeration. These effects typically result in chemical and/or physical entrapment of valuable metals and metallic compounds and possibly complicate subsequent leaching and purification steps for recovery [
43,
44]. The superior energetic advantage of incineration processes is hence typically established by giving up on the recovery of valuable organic products of thermal decomposition and, in some cases, metallic compounds of interest. However, through combustion of targeted mixed- or mono-waste, ash with high concentrations of valuable inorganics can still be produced, which are interesting substrates for hydrometallurgical extraction operations, e.g., Zn-rich ash from municipal solid waste [
45], Cu-rich ash from vineyard pruning wood [
46], Sb-rich ash from (brominated) flame-retardant plastics [
47], and P-rich ash from chicken manure [
48] and sewage sludge [
49,
50,
51].
Waste Gasification
Gasification is often put forward as an alternative to incineration and is perceived as more advanced in view of increasing the yield of organic chemicals from waste (e.g., C2–C4 compound gasses, oils, etc.) next to syngas for energetic purposes [
42,
52]. As gasification relies on sub-stoichiometric supply of O
2, the thermochemical reactions in the waste bed are ceased more rapidly than with incineration. For this reason, metallic compounds in the waste bed and solid residue are also less prone to oxidation, which could increase the recovery and valorization potential of the contained metals in some cases.
Despite these advantages, however, gasification processes cannot yet claim a proven track record in waste treatment on the industrial scale for a number of reasons, as set out in more detail in ref. [
5,
42,
52]. The major drawbacks of waste gasification include high selectivity in terms of waste input (which typically urges the use of mechanical pre-sorting equipment with moving parts that are prone to failure) and a strong dependence on a continuous supply of support energy to keep the gasification process going. The latter can either be supplied to the process through steam (e.g., from neighboring industries), gas/oil burners, or coal-based additives that generate thermochemical reaction heat in situ. In addition, gas engines (or turbines) that are possibly coupled to waste gasifiers are susceptible to fouling and abrasion by fly ash particles. This, in turn, urges flue gas treatment units to be operated at higher temperatures than with waste incinerators, which causes a number of complexities [
53] and leads to high capital expenditures.
These restrictions explain why waste gasifiers are still rare at the industrial scale and why they are, in reality, mostly conceived as two-staged thermal oxidizers operated in slightly over-stoichiometric O2 conditions (λ ≥ 1). In a two-staged process, sub-stoichiometric O2 conditions are typically established in the first stage, whilst (limited) excess of O2 is supplied in the second stage to burn the gasses generated in the first stage. Overall, oxidation with a net release of heat takes place, such that these processes can be regarded equally as de facto incinerators with a reduced λ-value (i.e., 1.0 to 1.1). However, compared to (full) waste incinerators, two-staged thermal oxidizers are compartmentalized and therefore subject to intrinsic limitations with regard to scale-up. This typically results in smaller waste throughput capacities, which makes it more difficult to establish a sufficient economy of scale.
2.2.3. Combustion Air: Carrier Flow in the Waste Bed
Due to the general use of the air-to-fuel ratio (λ), with 1 as sharp criterion value to distinguish, waste incineration and gasification are often perceived as opposite and mutually exclusive processes. However, this approach ignores the gas-solid complexity in typical waste combustion furnaces. Both incineration and gasification processes have thermal decomposition reactions in common, which are heterogeneous and take place in the interior of the waste particles. From a reaction-engineering perspective, these reactions are (to large extent) governed by intra-particle limitations of mass transfer (i.e., on transport of gaseous compounds) and heat transfer (i.e., on transport of heat required to initiate thermal decomposition) [
54,
55]. Then, with a slowed release of thermochemical reaction products from the waste particles, and with O
2 still largely unconsumed in the gas flowing around (and in between) the particles, oxidation reactions in the gas phase also become rate-limited.
The mechanisms of intra-particle mass and heat transfer (i.e., mass diffusion, thermal diffusion, and conduction) apply to both gasification and incineration processes, regardless of the λ-value applied at the overall process level. However, according to Arrhenius’ law, rates of reactions in the gas phase are expected to increase with increasing temperature. Hence, incineration processes (operated at relatively higher temperatures) are more rapidly affected by a shortage in the supply to the gas phase than gasification processes (operated at relatively lower temperatures). Deviations from (ideal) thermochemical predictions are then also expected to be relatively more significant in incineration than in gasification furnaces. The variety in aspect ratios and sizes of typical waste particles in mixed-waste streams and thermal losses due to evaporation of moisture also further increase these deviations.
Furthermore, similar to any heterogeneous reaction medium, reaction conditions inside a thermally decomposing waste bed are affected by physical aspects at the waste bed level, i.e., volume and height of the waste bed, temperature evolution across the bed, flow rate of the primary combustion air, and contact mode between waste particles and combustion air. Whereas waste beds in fluidized bed furnaces are highly homogenized in terms of temperature and compound concentrations, waste beds in moving grate furnaces are expected to behave more like packed-bed reactors subjected to very different conditions between top and bottom (
Figure 3). In terms of temperature, due to heat radiation from the freeboard above and supply of primary air (at about 15 to 250 °C) from the bottom, a temperature difference of up to 1000 °C is easily established across the waste bed. Hence, the rates of thermal decomposition and oxidation depend on the exact position in the waste bed, in both the vertical (i.e., different conditions of temperature) and horizontal (i.e., different residence time of the waste particles considered) directions.
Furthermore, when the pressure drop across the waste bed changes with bed height and/or flow rate of primary combustion air, convective mass transport in between particles in the waste bed is altered, which in turn affects reaction rates in the bed and concentrations of inorganic compounds possibly released. Recent observations from WtE combustion plants equipped with moving grates suggest that HCl and SO
2 concentrations in the generated combustion gasses can indeed be influenced through variating freeboard temperature, flow rate of primary combustion air, and waste bed height, as confirmed by ref. [
56,
57,
58]. However, similar results have not (yet) been reported by other authors.
Based on the principles elaborated above, a sharp distinction between incineration and gasification cannot be made at the overall process level, despite the (chemical) elegancy of the λ-based definition. Furthermore, as molecules and reactions do not scale up—unlike furnaces, particles, beds, and air flows—physical limitations on thermochemistry in waste furnaces are expected to become ever more critical with increasing scales of distance, volume, and time in plants of industrial size. This possibly explains why models previously mentioned in
Section 2.1.2., which aimed to predict the behavior of inorganic compounds based on chemical reactions and assumptions only (e.g., chemical-thermodynamic simulations), could not be validated in actual WtE combustion plants after all. The development of (reaction-) engineering models is further discussed in
Section 2.4.2.
2.3. Operational Aspects
2.3.1. Combustion Control in WtE Combustion Plants (Moving-Grate Furnace)
Basic Control Loops
A typical scheme for basic combustion control in WtE plants equipped with moving grates is shown in
Figure 4. In reality, control schemes implemented in WtE combustion plants are more complex, and include additional features and control loops that are supplier-specific, e.g., [
59,
60]. Waste combustion control is essentially based on three process values: generated steam flow (i.e., m’
st), waste throughput (i.e., m’
w), and excess O
2 (i.e., %
O2), with the latter regarded as an imposed constraint to the overall efficiency of combustion [
61,
62]. The main objective of combustion control is to steer the combustion of the waste at all times such that the steam flow generated is maximal (i.e., at the steam setpoint, SP m’
st) for a fixed setpoint value of excess O
2 (i.e., SP %
O2, typically between 4 and 8%v).
To achieve the main control objective, the height and distribution of the waste bed are monitored by continuous evaluation of process values mainly obtained from differential pressure measurements in the different combustion air flows and from visual and/or IR cameras viewing the waste bed (to observe the distribution of the waste and/or the heat release on the grate surface). The combustion control then continuously acts on the primary and secondary airflows supplied to the furnace (i.e., V’PA and V’SA), and on their distribution throughout the waste bed and freeboard. This control action depends on the setpoints SP V’air and SP AR, which respectively determine the (desired) total flow of combustion air (V’air) and the (desired) air ratio between primary and secondary combustion air (AR). These setpoints are typically changed automatically, together with the main setpoints for steam and excess O2. The control system also acts on the hydraulic drives of the grate system to push the waste forward and adapt the (relative) speeds of the different grate zones (i.e., u1–u4) to (re-)distribute the waste along the grate.
Stability of Combustion
Due to the heterogeneity of typical thermally treated waste, the actual energetic input to waste furnaces is rather unpredictable. Short-term fluctuations in LHV of the incoming waste can occur, which are mainly due to variable moisture content. The feeding mechanism of the waste into the furnace is then accelerated or slowed down by the control system in order to alter the waste throughput such that the steam flow produced is kept within a defined range around the imposed steam setpoint. Additionally, long-term fluctuations occur in LHV of the incoming waste due to more fundamental changes in the incoming waste (e.g., chemical composition of the combustible fraction). Then, the steam setpoint (i.e., SP m’st) requires adjustment throughout time, either manually (i.e., by the operator) or automatically (i.e., by steam setpoint tracking).
Overall, to keep compliance, on average, with the main setpoints (i.e., for steam flow, waste throughput and excess oxygen), the actions induced by combustion control in WtE furnaces continuously change the physical conditions that govern thermochemical reactions in the waste bed (
Section 2.2.3). Furthermore, the stability of the generated steam flow is controlled by additional and fast control on steam de-superheaters in the boiler (not shown in
Figure 4). In this way, the generated steam complies with industrial standards for application as process steam and damage to steam turbine generators coupled to WtE boilers is avoided.
Control Logics
The basic principles of combustion control have remained very similar to those applied in earlier times in coal-fired power plants, although the hardware has evolved considerably. Typical control in WtE combustion plants is still largely based on proportional-integral-differential (PID) control theory, which is well established and long-proven in process industries [
63]. However, PID control schemes are, in general, strongly reliant on feedback loops, which introduce delays in responsive actions of the control. Nevertheless, such control schemes are still mostly favored, as they act in line with human intuition and thus allow manual overrule of the control relatively easily, such as when unexpected events occur in plant operation (e.g., sudden failures of key electrical, mechanical, or hydraulic equipment). This advantage is obvious in WtE combustion plants due to the heavy-duty type of operation and possible consequences of exceeding legally imposed emission limits.
Today, predictive control systems based on data-driven models are slowly gaining ground also in the WtE industry [
64]. As these control systems, unlike PID-based systems, can reject data disturbances upfront, they are—in principle—able to establish and maintain better operational stability. In contrast, they are less comprehensible and accessible for plant operators and not yet widely proven in WtE combustion plants. All in all, due to the operation risk associated to (fully) predictive data-driven control, hybrid type combustion control systems, e.g., PID-based control systems with data-driven prediction of setpoint values, are perhaps an interesting option for WtE combustion processes.
2.3.2. The WtE Control Dilemma: Energy Efficiency versus Plant Availability
Recently built WtE combustion plants show thermodynamic adaptations to better utilize the energy produced, e.g., the recovery of low-temperature heat and the implementation of combined heat and power cycles. With regard to operation and control, the focus is strongly set on further stabilizing the generated steam flow since this enables a more direct—and thus, more efficient—application of the steam (e.g., as process steam for industrial users [
12]). However, as superheater corrosion remains a significant hurdle to date, the temperature of the superheated steam generated in WtE boilers must be kept limited below 420°C (i.e., the state-of-the art reference steam temperature for stand-alone WtE combustion plants). Temperatures above 420 °C come with a strong increase in capital and operational expenditures due to additional design features and are thus affordable only for WtE plant realizations with a strong business case [
5].
Finding the appropriate WtE combustion control settings to obtain maximum energy efficiency without sacrificing yearly plant availability is not a straightforward task.
Table 1 lists some of the combustion control objectives that are nowadays regularly pursued in WtE combustion plants equipped with moving grate furnaces and describes the impact of these objectives on energy efficiency and plant availability.
For example, in view of generating a highly stable steam flow in the boiler, a high(er) waste bed on the grate is regarded (and practically proven) as advantageous. Indeed, due to an increased flow resistance to the primary combustion air in the waste bed, fluctuations in the flow of the combustion gas generated in the furnace are better stabilized, and so, the heat exchange and steam production in the boiler downstream are less disturbed. According to industrial practice in WtE combustion plants, this advantage becomes more pronounced as more of the (total) combustion air is supplied as primary combustion air and less as secondary (or tertiary) air. Nevertheless, from a chemical perspective, O
2 supplied by primary combustion air but not consumed in the waste bed ends up in the freeboard, where it further reacts as if it were supplied by secondary combustion air. Hence, the exact source of O
2 is irrelevant in this regard, as long as the minimum O
2 requirement for primary combustion is at least fulfilled. However, from an engineering perspective, combustion air that could have been supplied directly to the freeboard (i.e., as secondary combustion air) instead of from below (i.e., as primary combustion air) can transfer a supplementary load of inorganic compounds (and ash particles) into the generated gas (as discussed in
Section 2.2.3).
This reasoning helps to understand why operational conditions that accelerate slagging, fouling, and corrosion by inorganic compounds are often provoked in WtE combustion furnaces and boilers when combustion control parameters are set with a (too) one-sided aim to establish high energy efficiency.
2.4. Process Intelligence Aspects
2.4.1. Sensors and Analytic Monitoring
As previously explained, concentrations of HCl and SO2—next to CO and CO2—in combustion gasses from waste furnaces can probably be controlled more proactively to better anticipate problems of slagging, fouling, corrosion, and excessive consumption of sorbents and chemicals in WtE combustion plants. However, in-line measured data on HCl and SO2 concentrations in combustion gasses—not yet available in most cases—are commonly used only for control of dosing of sorbents and chemicals in the flue gas treatment sections downstream, but disregarded for combustion control in furnaces upstream.
With regard to metallic compounds, the impact of fouling and corrosion can be monitored in-line, although with alternating success. For this purpose, electrochemical measurement techniques are applied on surfaces of boiler membrane walls or tubular steel probes inserted in the boiler [
65]. Direct techniques, such as pyro-acoustic measurements, are also applied [
66]. However, methods for direct in-line analysis of metallic vapors in combustion gasses [
67,
68] have not yet been (sufficiently) developed and validated for application in the challenging conditions that prevail in waste furnaces and boilers (e.g., dust, smoke, temperature, heat radiation, etc.) and may not be feasible. Finally, continuous characterization of the incoming waste—as already partially developed for (homogeneous) biomass fuels [
69]—is not straightforward due to the broadly varying compositions of typical (mixed) waste to be combusted.
2.4.2. Predictive Modeling
The need for in-line analytic measurements in WtE combustion plants can be strongly reduced by the development of predictive numerical models. Such models could be applied for soft sensor and advanced combustion control purposes in WtE combustion plants, provided a representative validation (e.g., pilot plants) with ever better developed analyzers that target inorganic compounds in solids and gasses.
Engineering Models
Engineering models based on computational fluid dynamics (CFD) are mostly used to gain deep insight into the complex interactions between thermochemistry, flow, and mass and heat transfer in combustion processes. Advanced CFD models, in both full-extensive as well as surrogate forms, were established for combustion of municipal solid waste and sewage sludge [
70,
71,
72,
73]. The literature shows, however, that the existing CFD models to date are still largely unable to predict the fate of inorganic compounds, in particular, and moreover, they lack experimental validation [
74,
75].
In general, CFD models are computationally heavy and as such, they are not well-suited for real-time control applications. Models of reduced-order, which combine fast computation with reasonable accuracy, therefore offer an attractive alternative. A particular class of reduced-order models comprises so-called compartmental reactor models, often referred to as a reactor network models (RNM). In such models, entirely based on reactor engineering principles, a network of idealized reactors/compartments is constructed to mimic complex reactive phenomena and processes [
76,
77]. Despite their great potential, however, models of this type are still largely nonexistent for thermal waste treatment, in particular, and need to be further established [
78].
Data-Driven Models
Finally, predictive data models based on actual industrial process data and/or simulated data from engineering models are also very promising—but yet unproven—in the field of waste combustion [
79,
80]. Depending on the modeling method applied, a numerical model emerges that is either more causal or more descriptive. For example, machine-learning models based on neural networks can deal with large amounts of (noisy) process data and allow accurate modeling of non-linear process behavior, but they still lack interpretability to allow for a fundamental interpretation of the obtained results. Sequence-based deep learning models, such as recurrent neural networks or temporal convolutional networks, are considered highly relevant for this time series prediction task. On the other hand, e.g., in tensor-based models [
81], process data can be regarded as a combination of a (limited) number of simple components having some low-rank structure that can be unraveled numerically to obtain predictive models that are physically and/or chemically meaningful.
2.5. Plant-Economical Aspects
To date, the prevention of boiler corrosion in WtE combustion plants has relied almost entirely on the application of corrosion-resistant layers on boiler tubes and walls (i.e., welded overlays and sprayed coatings) and cleaning systems that are typically based on a mechanical principle of action (e.g., acoustic waves, shock explosion, water shower, bullet shooting, hammering, etc.). Whilst this approach has proven so far effective in preventing the build-up of fouling deposits, it is not without risk of mechanical damage in case of improper use, nor does it take away the root cause of fouling.
In the European WtE industry, fouling and corrosion still rank second in terms of operating expenditures [
82].
Table 2 provides estimated values for direct and avoided costs and missed revenues due to the resulting non-availability of a single combustion line with a reference plant availability of 8000 h/year. (Bottom ash treatment is regarded as cost-neutral and is therefore not included.) The values are based on averages of market-compliant figures per ton of waste treated, which were kindly provided by several operating companies (which cannot be disclosed for reasons of confidentiality) [
82].
In
Table 2, indirect costs, i.e., costs due to compensations for losses and damages to third parties, are not included. Additionally, capital expenditures (i.e., depreciations of investment) are not considered since they are highly dependent on location, plant scale (i.e., number and waste throughput capacity of the combustion lines built), and contractual arrangements between the different parties typically involved in the investment, construction, and long-term operation of the WtE combustion plant.
Furthermore, the figures in
Table 2 are valid for a so-called stand-alone WtE combustion plant, i.e., a plant that typically produces electricity only. This plant concept is economically well understood from many years of experience. However, WtE plants are nowadays often built as highly flexible combined heat and power (CHP) plants that deliver energy in different forms to several residential, public, and industrial clients. These plant references come with complex, tailored designs [
5,
6], for which case-specific simulations (out of the scope of this paper) are required to assess the full economic loss in case of unplanned downtime. Nevertheless, it is clear that the typical revenues of WtE-CHP plants, and financial liabilities towards energy clients and parties involved, are significantly higher than for stand-alone WtE plants, such that the overall calculated loss due to unscheduled downtime can be regarded as a minimum scenario.
According to common industrial practice in an average WtE combustion plant, a maximum of two weeks in total is tolerated for unscheduled downtime per year. Reducing this downtime by about one week implies a direct yearly savings of approximately 330,000 EUR. Furthermore, on the assumption that up to 10% savings on sorbents and chemicals for flue gas treatment can be potentially realized by advanced combustion control, an additional yearly gain of up to 240,000 EUR per year (i.e., 8000 operation hours) can be established. Finally, the yearly cost associated with inspection and manual cleaning of the boiler is expected to be reduced by approximately 100,000 EUR, based on the experience in WtE combustion plants. Summing up all items, the potential economic loss due to downtime runs up to (at least) 670,000 EUR per year and per combustion line. Hence, the potential cost savings that can be established by implementing combustion control with further advanced thermochemical intelligence towards inorganic compounds is very significant.
3. Discussion
The overview in
Section 2 uncovers the overall difficulty to conciliate targets of high energy efficiency and high plant availability in state-of-the-art WtE combustion plants, due to inorganic compounds typically present in thermally treated waste. Operation and design requirements to obtain high energy efficiency, when taken too far, provoke operational inconvenience and damage, such as large fluctuations of inorganic pollutants in the generated combustion gasses, unpredictable fouling, and corrosion phenomena.
To date, scientific research has mainly tried to explain the behavior of inorganic compounds in terms of their concentrations in the treated waste, and results from experiments and models that heavily rely on idealized inorganic thermochemical interpretations. On the other hand, observations from industrial practice in WtE plants indicate that issues associated with inorganic compounds can be influenced by controlling the physical phenomena of flow and mass transport in and around the waste present in combustion furnaces. This is supported by many—although hardly documented—cases of deficient combustion control in industrial WtE combustion plants. The existing gap between current industrial knowledge in the field of WtE and scientific knowledge so far established is explained in
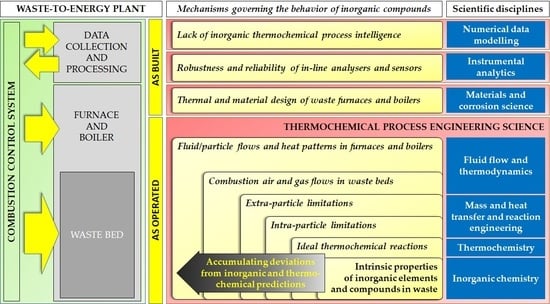
Figure 5 below.
As can be noticed from
Figure 5, scientific knowledge with regard to the behavior of inorganic compounds is largely situated at the bottom level, i.e., researchers have mainly tried to explain the fate of inorganic compounds in WtE combustion processes in terms of
thermochemical reactions. Conversely, models and experimental data particularly linking the behavior of inorganic compounds to physical conditions of mass and heat transfer (i.e.,
thermochemical reaction engineering) and overall fluid flow and solid-gas modes of contact between solids and gasses (i.e.,
thermochemical process engineering) remain uncommon to date. Additionally, lab-scale waste combustion experiments for scientific purposes have typically been conducted under physical conditions that are not representative of industrial furnaces (e.g., limited batches of small waste particles in stagnant environments of bench-scale muffle furnaces versus large waste pieces in turbulent flow-through regime in waste beds of industrial waste furnaces).
This likely explains the large discrepancies that commonly occur among predictions by (idealized) thermochemical models, results from experiments established with lab- and pilot-scale furnaces, and findings from full-scale WtE combustion plants (i.e.,
thermochemical process operation). Therefore, the actual release and transfer mechanisms of inorganic compounds in industrial waste combustion furnaces, especially in relation to continuously changing physical conditions induced by combustion control systems, are still largely unraveled to date. Overall, the topic of inorganic compound behavior is not fully understood scientifically, in particular from a higher level’s perspective (as shown in
Figure 5). Conversely, knowledge in the WtE industry with regard to inorganic compounds is mainly situated on the higher level and is mainly built on practical experience and qualitative observations. Hence, the main challenges for further scientific research and industrial innovation are clearly at intermediate levels. However, bridging this knowledge gap requires researchers with strong technical affinity on the one side and plant operators with experimental/scientific mindsets on the other.
In state-of-the-art WtE plants, combustion control is dedicated almost exclusively to the production of high and stable energy output, and thus to control of the carbon-related thermochemistry taking place in waste furnaces. However, due to a lack of control intelligence with respect to inorganic thermochemistry, the partitioning and speciation behavior of inorganic compounds cannot yet be controlled preventively in the combustion stage and still needs to be tackled almost fully with mechanical cleaning solutions and flue gas treatment processes downstream, i.e., a mainly curative approach. This lack of thermochemical process intelligence causes significant plant-economic losses and complicates the further increase in energy efficiency, optimization of flue gas treatment processes, and development of mono-combustion processes for the recovery of critical compounds that are targeted in the circular economy.
4. Conclusions
The presence of inorganic compounds, among others, turns materials and consumer goods at end-of-life, and contaminated substances, into non-recyclable waste due to the environmental and human health hazards they pose. Hereby, inorganic compounds that have formerly contributed to improved production, utilization, and/or longevity of materials, paradoxically become a barrier to further recycling and force the thermal treatment of the waste caused this way.
In view of developing combustion control schemes with more advanced thermochemical intelligence, focus needs to be set on unraveling the impact of flow and mass and heat transfer on the partitioning and speciation of inorganic compounds in the solid-gas environments of waste combustion furnaces. This urges research from a reaction and process engineering perspective, in particular, to complement the existing (and vast) scientific knowledge long since established on the thermochemical properties and reactions of inorganic compounds. Furthermore, engineering and data-driven numerical models still largely need to be developed, to feed control systems in WtE combustion plants with dedicated process intelligence towards inorganic compounds that are typically present in thermally treated wastes.
All of this is ultimately indispensable to meet the growing demand for energy and critical materials recovered from unrecyclable waste streams in the circular economy.