Efficiency of Chemical Pretreatment of Sugar Beet Pulp Biomass Intended to Energy Production via Biological Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Examined Materials
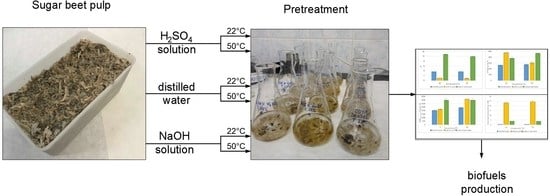
2.2. Sugar Beet Pulp Pretreatment
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results
3.1. The pH of the Hydrolysates
3.2. Electrolytic Conductivity of the Hydrolysates
3.3. COD of the Hydrolysates
3.4. VFA of the Hydrolysates
3.5. Concentration of Phenols in the Hydrolysates
4. Discussion
5. Conclusions
- The solubilizing effect of these pretreatment methods depended on temperature, even at its low range, as shown by the similar solubilization efficiency of beet pulp biomass obtained as a result of alkaline pretreatment (4% NaOH w/w) at 22 °C and after pretreating it with acid (10% H2SO4) at 50 °C.
- To obtain a comparable solubilization effect, it would be more advisable to use the alkaline pretreatment at 22 °C due to its lower costs. The reduction of costs results from savings on heating and lower consumption of NaOH than H2SO4 taking into account the unit mass of the pulp (the consumption of NaOH is 2.5 times lower, while the price for 1 kg of both reactants is similar).
- Under alkaline pretreatment at a lower temperature, the concentration of volatile fatty acids was lower, both when compared with the alkaline pretreatment carried out at 50 °C and the acid treatment at 22 °C.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marks-Bielska, R.; Bielski, S.; Novikova, A.; Romaneckas, K. Straw Stocks as a Source of Renewable Energy. A Case Study of a District in Poland. Sustainability 2019, 11, 4714. [Google Scholar] [CrossRef] [Green Version]
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change-A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, A.; Crane, A. Addressing sustainability and consumption. J. Macromarketing 2005, 25, 76–92. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sust. Energ. Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Qian, X. Statistical Analysis and Evaluation of the Advanced Biomass and Natural Gas Co-Combustion Performance. Ph.D. Thesis, Morgan State University, Baltimore, MD, USA, 2019. [Google Scholar]
- Demirbas, A. Potential applications of renewable energy sources, biomass combustion problems in boiler power systems and combustion related environmental issues. Prog. Energy Combust. Sci. 2005, 31, 171–192. [Google Scholar] [CrossRef]
- Masud, M.H.; Ananno, A.A.; Arefin, A.M.E.; Ahamed, R.; Das, P.; Joardder, M.U.H. Perspective of biomass energy conversion in Bangladesh. Clean Techn. Environ. Policy 2019, 21, 719–731. [Google Scholar] [CrossRef]
- Saidur, R.; Abdelaziz, E.A.; Demirbas, A.; Hossain, M.S.; Mekhilef, S. A review on biomass as a fuel for boilers. Renew. Sust. Energ. Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Cong, W.-F.; Moset, V.; Feng, L.; Møller, H.B.; Eriksen, J. Anaerobic co-digestion of grass and forbs—Influence of cattle manure or grass based inoculum. Biomass Bioenergy 2018, 119, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Decker, S.R.; Sheehan, J.; Dayton, D.C.; Bozell, J.J.; Adney, W.S.; Hames, B.; Thomas, S.R.; Bain, R.L.; Czernik, S.; Zhang, M.; et al. Biomass conversion. In Handbook of Industrial Chemistry and Biotechnology; Springer: Boston, MA, USA, 2012; pp. 1249–1322. [Google Scholar]
- Das, S.K.; Majhi, S.; Mohanty, P.; Pant, K.K. CO-hydrogenation of syngas to fuel using silica supported Fe–Cu–K catalysts: Effects of active components. Fuel Proc. Technol. 2014, 118, 82–89. [Google Scholar] [CrossRef]
- Saxena, R.C.; Adhikari, D.K.; Goyal, H.B. Biomass-based energy fuel through biochemical routes: A review. Renew. Sust. Energ. Rev. 2009, 13, 167–178. [Google Scholar] [CrossRef]
- Frigon, J.C.; Guiot, S.R. Biomethane production from starch and lignocellulosic crops: A comparative review. Biofuel. Bioprod. Biorefin. 2010, 4, 447–458. [Google Scholar] [CrossRef] [Green Version]
- Monroy, O.; Famà, G.; Meraz, M.; Montoya, L.; Macarie, H. Anaerobic digestion for wastewater treatment in Mexico: State of the technology. Water. Res. 2000, 34, 1803–1816. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Azarbaijani, R.; Parsa Yeganeh, L.; Angelidaki, I.; Nizami, A.S.; Bhat, R.; Dashora, K.; Vijay, V.K.; Aghbashlo, M.; et al. Pretreatment of lignocelluloses for enhanced biogas production: A review on influencing mechanisms and the importance of microbial diversity. Renew. Sust. Energ. Rev. 2021, 135, 110173. [Google Scholar] [CrossRef]
- Moiser, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Karki, B.; Maurer, D.; Jung, S. Efficiency of pretreatments for optimal enzymatic saccharification of soybean fiber. Bioresour. Technol. 2011, 102, 6522–6528. [Google Scholar] [CrossRef]
- Song, Z.; Yang, G.; Liu, X.; Yan, Z.; Yuan, Y.; Liao, Y. Comparison of seven chemical pretreatments of corn straw for improving methane yield by anaerobic digestion. PLoS ONE 2014, 9, e93801. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Y.; Dong, Y. Pretreatment for biogas production by anaerobic fermentation of mixed corn stover and cow dung. Energy 2012, 46, 644–648. [Google Scholar] [CrossRef]
- Tarkow, H.; Feist, W.C. A mechanism for improving the digestibility of lignocellulosic materials with dilute alkali and liquid ammonia. In Cellulases and Their Applications; Gould, R.F., Ed.; American Chemical Society: Washington, DC, USA, 1969; pp. 197–218. [Google Scholar]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Kim, I.; Han, J. Optimization of alkaline pretreatment conditions for enhancing glucose yield of rice straw by response surface methodology. Biomass Bioenergy 2012, 46, 210–217. [Google Scholar] [CrossRef]
- Saha, B.C.; Iten, L.B.; Cotta, M.A.; Wu, Y.V. Dilute acid pretreatment, enzymatic saccharification, and fermentation of wheat straw to ethanol. Proc. Biochem. 2005, 40, 3693–3700. [Google Scholar] [CrossRef]
- Zheng, Y.; Lee, C.; Yu, C.; Cheng, Y.-S.; Zhang, R.; Jenkins, B.M.; VanderGheynst, J.S. Dilute acid pretreatment and fermentation of sugar beet pulp to ethanol. Appl. Energy 2013, 105, 1–7. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Wang, W.; Grifn, J.; Roozeboom, K.; Wang, D. Bioconversion of industrial hemp biomass for bioethanol production: A review. Fuel 2020, 281, 118725. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Banks, C.J.; Lo, H.-M. Assessing the effects of municipal solid waste incinerator bottom ash on the decomposition of biodegradable waste using a completely mixed anaerobic reactor. Waste Manage. Res. 2003, 21, 225–234. [Google Scholar] [CrossRef]
- Wang, Z.; Keshwani, D.R.; Redding, A.P.; Cheng, J.J.J.B. Sodium hydroxide pretreatment and enzymatic hydrolysis of coastal Bermuda grass. Bioresour. Technol. 2010, 101, 3583–3585. [Google Scholar] [CrossRef] [Green Version]
- Spagnuolo, M.; Crecchio, C.; Pizzigallo, M.D.R.; Ruggiero, P. Synergistic effects of cellulolytic and pectinolytic enzymes in degrading sugar beet pulp. Bioresour. Technol. 1997, 60, 215–222. [Google Scholar] [CrossRef]
- Kühnel, S.; Schols, H.A.; Gruppen, H. Aiming for the complete utilization of sugar-beet pulp: Examination of the effects of mild acid and hydrothermal pretreatment followed by enzymatic digestion. Biotechnol. Biofuels 2011, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Michel, F.; Thibault, J.F.; Barry, J.L. Preparation and characterisation of dietary fibre from sugar beet pulp. J. Sci. Food Agric. 1988, 42, 77–85. [Google Scholar] [CrossRef]
- Micard, V.; Renard, C.M.; Thibault, J.F. Enzymatic saccharification of sugar-beet pulp. Enzyme Microb. Technol. 1996, 19, 162–170. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C. Valorization of sugar beet pulp through biotechnological approaches: Recent developments. Biotechnol. Lett. 2021, 43, 1253–1263. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Diwan, D.; Tripathi, M.; Whale, E.; Jayakody, L.N.; Moreau, B.; Thakur, V.K.; Tuohy, M.; Gupta, V.K. Valorization of sugar beet pulp to value-added products: A review. Bioresour. Technol. 2022, 346, 126580. [Google Scholar] [CrossRef] [PubMed]
- Özbaș, K.E.; Özbaș, Ö.Ö. Sugar beet pulp as biomass. Sugar Industry 2017, 142, 29–32. [Google Scholar] [CrossRef]
- Kamzon, M.A.; Abderafi, S. Simulation study testing sulfuric acid pretreatment and hydrolysis of bagasse and beet pulp, to produce bioethanol in the Moroccan sugar industry. In Proceedings of the 2017 International Renewable and Sustainable Energy Conference (IRSEC), Tangier, Morocco, 4–7 December 2017; pp. 1–5. [Google Scholar]
- Grabarczyk, R.; Urbaniec, K.; Koukios, E.; Bakker, R.; Vaccari, G. Options of sugar beet pretreatment for hydrogen fermentation. Sugar Ind. 2011, 136, 784–790. [Google Scholar] [CrossRef]
- Gönen, C.; Akter Önal, N.; Deveci, E.Ü. Optimization of sugar beet pulp pre-treatment with weak and strong acid under pressure and non-pressure conditions via RSM. Biomass Convers. Biorefin. 2022, 1–4. [Google Scholar] [CrossRef]
- Kharina, M.; Emelyanov, V.; Mokshina, N.; Ibragimova, N.; Gorshkova, T. Pretreatment of sugar beet pulp with dilute sulfurous acid is effective for multipurpose usage of carbohydrates. Appl. Biochem. Biotechnol. 2016, 179, 307–320. [Google Scholar] [CrossRef]
- Ziemiński, K.; Kowalska-Wentel, M. Effect of different sugar beet pulp pretreatments on biogas production efficiency. Appl. Biochem. Biotechnol. 2017, 181, 1211–1227. [Google Scholar] [CrossRef] [Green Version]
- Şenol, H.; Açıkel, Ü.; Oda, V. Anaerobic digestion of sugar beet pulp after acid thermal and alkali thermal pretreatments. Biomass Convers. Biorefin. 2021, 11, 895–905. [Google Scholar] [CrossRef]
- Chen, Y.; Stevens, M.A.; Zhu, Y.; Holmes, J.; Xu, H. Understanding of alkaline pretreatment parameters for corn stover enzymatic saccharification. Biotechnol. Biofuels 2013, 6, 8. [Google Scholar] [CrossRef]
- Mohamad Remli, N.A.; Md Shah, U.K.; Mohamad, R.; Abd-Aziz, S. Effects of chemical and thermal pretreatments on the enzymatic saccharification of rice straw for sugars production. BioResources. 2014, 9, 510–522. [Google Scholar] [CrossRef] [Green Version]
- Raina, N.; Slathia, P.S.; Sharma, P. Experimental optimization of thermochemical pretreatment of sal (Shorea robusta) sawdust by Central Composite Design study for bioethanol production by co-fermentation using Saccharomyces cerevisiae (MTCC-36) and Pichia stipitis (NCIM-3498). Biomass Bioenergy 2020, 143, 105819. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Gómez, L.D.; Wei, T.; Yang, X.; Simister, R.; McQueen-Mason, S.J.; Macquarrie, D.J. Thermochemical pretreatments of maize stem for sugar recovery: Comparative evaluation of microwave and conventional heating. Ind. Crops Prod. 2021, 160, 113106. [Google Scholar] [CrossRef]
- Kang, X.; Zhang, Y.; Song, B.; Sun, Y.; Li, L.; He, Y.; Kong, X.; Luo, X.; Yuan, Z. The effect of mechanical pretreatment on the anaerobic digestion of Hybrid Pennisetum. Fuel 2019, 252, 469–474. [Google Scholar] [CrossRef]
- Ozkan, L.; Erguder, T.H.; Demirer, G.N. Effects of pretreatment methods on solubilization of beet-pulp and bio-hydrogen production yield. Int. J. Hydrog. Energy 2011, 36, 382–389. [Google Scholar] [CrossRef]
- Ziemiński, K.; Romanowska, I.; Kowalska-Wentel, M.; Cyran, M. Effects of hydrothermal pretreatment of sugar beet pulp for methane production. Bioresour. Technol. 2014, 166, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Statista. Sugar Production from Sugar Beet in Selected European Countries from 2018/19 to 2020/21. Available online: https://www.statista.com/statistics/1126827/sugar-beet-sugar-production-in-european-countries/ (accessed on 6 December 2022).
- Kassim, M.A.; Meng, T.K.; Kamaludin, R.; Hussain, A.H.; Nurul Adela Bukhari, N.A. Chapter 9—Bioprocessing of sustainable renewable biomass for bioethanol production. In Value-Chain of Biofuels; Yusup, S., Rashidi, N.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–234. [Google Scholar]
- Elalami, D.; Barakat, A. State of the art of energy production from agricultural residues using thermochemical and biological processes. In Clean Energy and Resources Recovery, Biomass Waste Based Biorefineries; Elsevier: Amsterdam, The Netherlands, 2021; Volume 1, pp. 1–24. [Google Scholar]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid hydrolysis of lignocellulosic biomass: Sugars and furfurals formation. Catalysts 2020, 10, 437. [Google Scholar] [CrossRef] [Green Version]
- Wagner, H. Influence of temperature on electrical conductivity of diluted aqueous solutions. Power Plant Chem. 2012, 14, 455–469. [Google Scholar]
- Nenkova, S.; Vasileva, T.; Stanulov, K. Production of phenol compounds by alkaline treatment of technical hydrolysis lignin and wood biomass. Chem. Nat. Compd. 2008, 44, 182–185. [Google Scholar] [CrossRef]
- Ares-Peón, I.A.; Garrote, G.; Domínguez, H.; Parajó, J.C. Phenolics production from alkaline hydrolysis of autohydrolysis liquors. CyTA-J. Food 2016, 14, 255–265. [Google Scholar] [CrossRef]
- Kayembe, K.; Basosila, L.; Mpiana, P.T.; Sikulisimwa, P.C.; Mbuyu, K. Inhibitory effects of phenolic monomers on methanogenesis in anaerobic digestion. Br. Microbiol. Res. J. 2013, 3, 32–41. [Google Scholar] [CrossRef]
- Wang, Y.T.; Suidan, M.T.; Pfeffer, J.T.; Najm, I. Effects of some alkyl phenols on methanogenic degradation of phenol. Appl. Environ. Microbiol. 1988, 54, 1277–1279. [Google Scholar] [CrossRef] [Green Version]
- Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions REPowerEU Plan COM/2022/230 final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2022%3A230%3AFIN&qid=1653033742483 (accessed on 30 December 2022).
- D’Adamo, I.; Ribichini, M.; Tsagarakis, K.P. Biomethane as an energy resource for achieving sustainable production: Economic assessments and policy implications. Sustain. Prod. Consum. 2023, 35, 13–27. [Google Scholar] [CrossRef]
- González-Castaño, M.; Kour, M.H.; González-Arias, J.; Baena-Moreno, F.M.; Arellano-Garcia, H. Promoting bioeconomy routes: From food waste to green biomethane. A profitability analysis based on a real case study in eastern Germany. J. Environ. Manag. 2021, 300, 113788. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Unit | Value |
|---|---|---|
| Total solids (TS) | % | 93.35 ± 3.51 |
| Volatile solids (VS) | % d.w. | 94.56 ± 0.69 |
| COD | mgO2 g d.w.−1 | 1831 ± 169 |
| C | % d.w. | 40.09 ± 0.53 |
| H | % d.w. | 4.8 ± 0.01 |
| N | % d.w. | 1.57 ± 0.04 |
| S | % d.w. | 0.102 ± 0.003 |
| Ash | % d.w. | 5.22 ± 0.15 |
| Fixed carbon | % d.w. | 0.22 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawłowska, M.; Zdeb, M.; Nieścioruk, M. Efficiency of Chemical Pretreatment of Sugar Beet Pulp Biomass Intended to Energy Production via Biological Processes. Energies 2023, 16, 574. https://doi.org/10.3390/en16020574
Pawłowska M, Zdeb M, Nieścioruk M. Efficiency of Chemical Pretreatment of Sugar Beet Pulp Biomass Intended to Energy Production via Biological Processes. Energies. 2023; 16(2):574. https://doi.org/10.3390/en16020574
Chicago/Turabian StylePawłowska, Małgorzata, Magdalena Zdeb, and Monika Nieścioruk. 2023. "Efficiency of Chemical Pretreatment of Sugar Beet Pulp Biomass Intended to Energy Production via Biological Processes" Energies 16, no. 2: 574. https://doi.org/10.3390/en16020574