Polyaniline-Based Ink for Inkjet Printing for Supercapacitors, Sensors, and Electrochromic Devices
Abstract
:1. Introduction
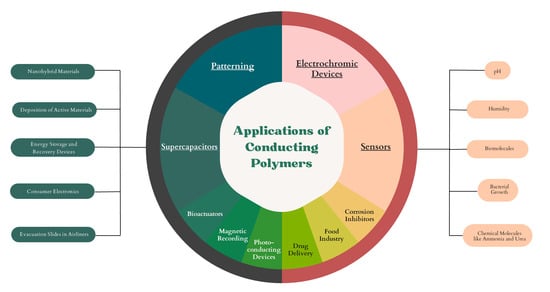
2. Applications of Inkjet Printer Conducting Polymers
2.1. Conductive Polymer for Supercapacitors
2.2. Conductive Polymers for Sensors
2.3. Conductive Polymers for Electrochromic Devices
2.4. Patterning with Conductive Polymers
3. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oliveira, J.; Correia, V.; Castro, H.; Martins, P.; Lanceros-Mendez, S. Polymer-Based Smart Materials by Printing Technologies: Improving Application and Integration. Addit. Manuf. 2018, 21, 269–283. [Google Scholar] [CrossRef]
- Wu, S.; Zeng, T.; Liu, Z.; Ma, G.; Xiong, Z.; Zuo, L.; Zhou, Z. 3D Printing Technology for Smart Clothing: A Topic Review. Materials 2022, 15, 7391. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Felipe, C.; Oliveira, J.; Etxebarria, I.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. State-of-the-Art and Future Challenges of UV Curable Polymer-Based Smart Materials for Printing Technologies. Adv. Mater. Technol. 2019, 4, 1800618. [Google Scholar] [CrossRef]
- Borghetti, M.; Cantù, E.; Sardini, E.; Serpelloni, M. Future Sensors for Smart Objects by Printing Technologies in Industry 4.0 Scenario. Energies 2020, 13, 5916. [Google Scholar] [CrossRef]
- Kim, A.; Oh, S.H.; Adhikari, A.; Sathe, B.R.; Kumar, S.; Patel, R. Recent Advances in Modified Commercial Separators for Lithium–Sulfur Batteries. J. Mater. Chem. A 2023, 11, 7833–7866. [Google Scholar] [CrossRef]
- Patel, M.; Patel, R.; Park, C.; Cho, K.; Kumar, P.; Park, C.; Koh, W.-G. Water-Stable, Biocompatible, and Highly Luminescent Perovskite Nanocrystals-Embedded Fiber-Based Paper for Anti-Counterfeiting Applications. Nano Converg. 2023, 10, 21. [Google Scholar] [CrossRef]
- Kim, A.; Wert, N.A.; Gowd, E.B.; Patel, R. Recent Progress in PEG-Based Composite Phase Change Materials. Polym. Rev. 2023, 1–52. [Google Scholar] [CrossRef]
- Kim, A.; Dash, J.K.; Kumar, P.; Patel, R. Carbon-Based Quantum Dots for Photovoltaic Devices: A Review. ACS Appl. Electron. Mater. 2022, 4, 27–58. [Google Scholar] [CrossRef]
- Patel, M.; Meenu, M.; Pandey, J.K.; Kumar, P.; Patel, R. Recent Development in Upconversion Nanoparticles and Their Application in Optogenetics: A Review. J. Rare Earths 2022, 40, 847–861. [Google Scholar] [CrossRef]
- Gupta, B.K.; Kumar, P.; Kedawat, G.; Kanika; Vithayathil, S.A.; Gangwar, A.K.; Singh, S.; Kashyap, P.K.; Lahon, R.; Singh, V.N.; et al. Tunable Luminescence from Two Dimensional BCNO Nanophosphor for High-Contrast Cellular Imaging. RSC Adv. 2017, 7, 41486–41494. [Google Scholar] [CrossRef]
- Gupta, B.K.; Kedawat, G.; Kumar, P.; Singh, S.; Suryawanshi, S.R.; Agrawal (Garg), N.; Gupta, G.; Kim, A.R.; Gupta, R.K.; More, M.A.; et al. Field Emission Properties of Highly Ordered Low-Aspect Ratio Carbon Nanocup Arrays. RSC Adv. 2016, 6, 9932–9939. [Google Scholar] [CrossRef]
- Hrytsenko, O.; Hrytsenko, D.; Shvalagin, V.; Grodziuk, G.; Kompanets, M. The Use of Carbon Nanoparticles for Inkjet-Printed Functional Labels for Smart Packaging. J. Nanomater. 2018, 2018, 6485654. [Google Scholar] [CrossRef]
- Cirelli, M.; Hao, J.; Bor, T.C.; Duvigneau, J.; Benson, N.; Akkerman, R.; Hempenius, M.A.; Vancso, G.J. Printing “Smart” Inks of Redox-Responsive Organometallic Polymers on Microelectrode Arrays for Molecular Sensing. ACS Appl. Mater. Interfaces 2019, 11, 37060–37068. [Google Scholar] [CrossRef] [PubMed]
- Seipel, S.; Yu, J.; Periyasamy, A.P.; Viková, M.; Vik, M.; Nierstrasz, V.A. Inkjet Printing and UV-LED Curing of Photochromic Dyes for Functional and Smart Textile Applications. RSC Adv. 2018, 8, 28395–28404. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Seipel, S.; Nierstrasz, V.A. Digital Inkjet Functionalization of Water-Repellent Textile for Smart Textile Application. J. Mater. Sci. 2018, 53, 13216–13229. [Google Scholar] [CrossRef]
- Bocchini, S.; Chiolerio, A.; Porro, S.; Accardo, D.; Garino, N.; Bejtka, K.; Perrone, D.; Pirri, C.F. Synthesis of Polyaniline-Based Inks, Doping Thereof and Test Device Printing towards Electronic Applications. J. Mater. Chem. C 2013, 1, 5101–5109. [Google Scholar] [CrossRef]
- Nair, N.M.; Pakkathillam, J.K.; Kumar, K.; Arunachalam, K.; Ray, D.; Swaminathan, P. Printable Silver Nanowire and PEDOT:PSS Nanocomposite Ink for Flexible Transparent Conducting Applications. ACS Appl. Electron. Mater. 2020, 2, 1000–1010. [Google Scholar] [CrossRef]
- Singh, A.; Katiyar, M.; Garg, A. Understanding the Formation of PEDOT:PSS Films by Ink-Jet Printing for Organic Solar Cell Applications. RSC Adv. 2015, 5, 78677–78685. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Stoeckel, M.-A.; Ruoko, T.-P.; Wu, H.-Y.; Liu, X.; Kolhe, N.B.; Wu, Z.; Puttisong, Y.; Musumeci, C.; Massetti, M.; et al. A High-Conductivity n-Type Polymeric Ink for Printed Electronics. Nat. Commun. 2021, 12, 2354. [Google Scholar] [CrossRef]
- Liu, X.; Shen, Y.; Yang, R.; Zou, S.; Ji, X.; Shi, L.; Zhang, Y.; Liu, D.; Xiao, L.; Zheng, X.; et al. Inkjet Printing Assisted Synthesis of Multicomponent Mesoporous Metal Oxides for Ultrafast Catalyst Exploration. Nano Lett. 2012, 12, 5733–5739. [Google Scholar] [CrossRef]
- Negro, A.; Cherbuin, T.; Lutolf, M.P. 3D Inkjet Printing of Complex, Cell-Laden Hydrogel Structures. Sci. Rep. 2018, 8, 17099. [Google Scholar] [CrossRef]
- Willert, A.; Tabary, F.Z.; Zubkova, T.; Santangelo, P.E.; Romagnoli, M.; Baumann, R.R. Multilayer Additive Manufacturing of Catalyst-Coated Membranes for Polymer Electrolyte Membrane Fuel Cells by Inkjet Printing. Int. J. Hydrogen Energy 2022, 47, 20973–20986. [Google Scholar] [CrossRef]
- Hussain, A.; Abbas, N.; Ali, A. Inkjet Printing: A Viable Technology for Biosensor Fabrication. Chemosensors 2022, 10, 103. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Dominguez-Alfaro, A.; Lopez-Larrea, N.; Alegret, N.; Mecerreyes, D. Additive Manufacturing of Conducting Polymers: Recent Advances, Challenges, and Opportunities. ACS Appl. Polym. Mater. 2021, 3, 2865–2883. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of Electrically Conducting Organic Polymers: Halogen Derivatives of Polyacetylene, (CH). J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Shirakawa, H.; McDiarmid, A.; Heeger, A. Twenty-Five Years of Conducting Polymers. Chem. Commun. 2003, 1, 1–4. [Google Scholar] [CrossRef]
- Guo, X. Conducting Polymers Forward. Nat. Mater. 2020, 19, 921. [Google Scholar] [CrossRef]
- El-Bery, H.M.; Salah, M.R.; Ahmed, S.M.; Soliman, S.A. Efficient Non-Metal Based Conducting Polymers for Photocatalytic Hydrogen Production: Comparative Study between Polyaniline, Polypyrrole and PEDOT. RSC Adv. 2021, 11, 13229–13244. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Shoaie, I.S.; Khalilzadeh, M.A.; Asl, M.S.; Van Le, Q.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent Developments in Conducting Polymers: Applications for Electrochemistry. RSC Adv. 2020, 10, 37834–37856. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting Polymers: A Comprehensive Review on Recent Advances in Synthesis, Properties and Applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Benny Mattam, L.; Bijoy, A.; Abraham Thadathil, D.; George, L.; Varghese, A. Conducting Polymers: A Versatile Material for Biomedical Applications. ChemistrySelect 2022, 7, e202201765. [Google Scholar] [CrossRef]
- Atkare, S.; Hambir, S.; Jagtap, S.; Adhikari, A.; Singh, S.K.; Patel, R. Role of Polyaniline/Molybdenum Trioxide Nanocomposites in Tuning the Characteristics of Humidity Sensors. Polym. Adv. Technol. 2023, 34, 2585–2596. [Google Scholar] [CrossRef]
- Kumaravel, S.; Kim, E.; Kale, B.B.; Adhikari, A.; Patel, R.; Kundu, S. Recent Developments in Conductive Polymer-Based Electro-/Photoelectrocatalytic Materials for Effective Hydrogen/Oxygen Evolution Reactions: A Review. ChemElectroChem 2022, 9, e202200724. [Google Scholar] [CrossRef]
- Park, S.; Patel, R. Recent Progress in Conductive Polymer-Based Membranes. Membr. J. 2021, 31, 101–119. [Google Scholar] [CrossRef]
- Li, J.; Huckleby, A.B.; Zhang, M. Polymer-Based Thermoelectric Materials: A Review of Power Factor Improving Strategies. J. Mater. 2022, 8, 204–220. [Google Scholar] [CrossRef]
- Nasir, A.; Raza, A.; Tahir, M.; Yasin, T.; Nadeem, M.; Ahmad, B. Synthesis and Study of Polyaniline Grafted Graphene Oxide Nanohybrids. Mater. Res. Bull. 2023, 157, 112006. [Google Scholar] [CrossRef]
- Yao, F.; Xie, W.; Ma, C.; Wang, D.; El-Bahy, Z.M.; Helal, M.H.; Liu, H.; Du, A.; Guo, Z.; Gu, H. Superb Electromagnetic Shielding Polymer Nanocomposites Filled with 3-Dimensional p-Phenylenediamine/Aniline Copolymer Nanofibers@copper Foam Hybrid Nanofillers. Compos. Part B Eng. 2022, 245, 110236. [Google Scholar] [CrossRef]
- Dandapani; Devendra, K.; Revannasiddappa; Vishnu, K.R. Thermal Stability and Electromagnetic Interference of Epoxy-Graphene/Hybrid Composite Materials. Mater. Today Proc. 2022, 66, 1664–1670. [Google Scholar] [CrossRef]
- Thakur, Y.S.; Acharya, A.D.; Sharma, S. Bhawna Reinforcement of V2O5 Nanoparticle in Polyaniline to Improve the Optical and UV- Shielding Properties. Results Opt. 2023, 11, 100400. [Google Scholar] [CrossRef]
- Ray, B.; Parmar, S.; Date, K.; Datar, S. Optically Transparent Polymer Composites: A Study on the Influence of Filler/Dopant on Electromagnetic Interference Shielding Mechanism. J. Appl. Polym. Sci. 2021, 138, 50255. [Google Scholar] [CrossRef]
- Zhu, N.; Jiang, T.; Zeng, X.; Li, S.; Shen, C.; Zhang, C.; Gong, W.; He, L. High Strength and Light Weight Polyamide 6/Carbon Fiber Composite Foams for Electromagnetic Interference Shielding. J. Appl. Polym. Sci. 2023, 140, e53818. [Google Scholar] [CrossRef]
- Qi, H.; Wang, G.; Ma, Q.; Li, D.; Dong, X.; Yu, W.; Wang, J.; Liu, G.; Zhang, X. Conjugative Electrospinning towards Janus-Type Nanofibers Array Membrane Concurrently Displaying Dual-Functionality of Improved Red Luminescence and Tuneable Superparamagnetism. J. Mater. Sci. Mater. Electron. 2022, 33, 4438–4449. [Google Scholar] [CrossRef]
- Guo, X.; Xue, Z.; Zhang, Y. Manufacturing of 3D Multifunctional Microelectronic Devices: Challenges and Opportunities. NPG Asia Mater. 2019, 11, 29. [Google Scholar] [CrossRef]
- Satoh, Y.; Ding, H.; Yang, H.; Deng, Y.; Hsueh, A.-J.; Shimizu, T.; Qiao, M.; Ma, C.; Kariya, K.; Kurihara, T.; et al. Wired Microfabricated Electrochemical Systems. Anal. Chem. 2021, 93, 12655–12663. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Tan, L.; Regnier, F. Microfabricated Filters for Microfluidic Analytical Systems. Anal. Chem. 1999, 71, 1464–1468. [Google Scholar] [CrossRef]
- Grenci, G.; Bertocchi, C.; Ravasio, A. Integrating Microfabrication into Biological Investigations: The Benefits of Interdisciplinarity. Micromachines 2019, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chatrathi, M.P.; Tian, B.; Polsky, R. Microfabricated Electrophoresis Chips for Simultaneous Bioassays of Glucose, Uric Acid, Ascorbic Acid, and Acetaminophen. Anal. Chem. 2000, 72, 2514–2518. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Bhat, S.; Mahato, K.K. Design and Fabrication of Low-Cost Microfluidic Channel for Biomedical Application. Sci. Rep. 2020, 10, 9215. [Google Scholar] [CrossRef]
- Su, W.; Cook, B.S.; Fang, Y.; Tentzeris, M.M. Fully Inkjet-Printed Microfluidics: A Solution to Low-Cost Rapid Three-Dimensional Microfluidics Fabrication with Numerous Electrical and Sensing Applications. Sci. Rep. 2016, 6, 35111. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.H.; Mohammed, L.A.; Hammoodi, O.G.; Sehgal, S.; Alheety, M.A.; Saxena, K.K.; Dadoosh, S.A.; Mohammed, I.K.; Jasim, M.M.; Salmaan, N.U. A Review on Polyaniline: Synthesis, Properties, Nanocomposites, and Electrochemical Applications. Int. J. Polym. Sci. 2022, 2022, 9047554. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef] [PubMed]
- Park, J.T.; Patel, R.; Jeon, H.; Kim, D.J.; Shin, J.-S.; Hak Kim, J. Facile Fabrication of Vertically Aligned TiO2 Nanorods with High Density and Rutile/Anatase Phases on Transparent Conducting Glasses: High Efficiency Dye-Sensitized Solar Cells. J. Mater. Chem. 2012, 22, 6131–6138. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Development of Molecularly Imprinted Polymer Based Phase Boundaries for Sensors Design (Review). Adv. Colloid Interface Sci. 2022, 305, 102693. [Google Scholar] [CrossRef] [PubMed]
- Gerard, M.; Chaubey, A.; Malhotra, B.D. Application of Conducting Polymers to Biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.I.; Lee, S.B. Fast Electrochemistry of Conductive Polymer Nanotubes: Synthesis, Mechanism, and Application. Acc. Chem. Res. 2008, 41, 699–707. [Google Scholar] [CrossRef]
- Ahmad, K.; Raza, W. Current State and Prospective of Supercapacitors BT-Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Torres-Martínez, L.M., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1835–1853. ISBN 978-3-030-36268-3. [Google Scholar]
- Chi, K.; Zhang, Z.; Xi, J.; Huang, Y.; Xiao, F.; Wang, S.; Liu, Y. Freestanding Graphene Paper Supported Three-Dimensional Porous Graphene-Polyaniline Nanocomposite Synthesized by Inkjet Printing and in Flexible All-Solid-State Supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 16312–16319. [Google Scholar] [CrossRef]
- Chiolerio, A.; Bocchini, S.; Porro, S. Inkjet Printed Negative Supercapacitors: Synthesis of Polyaniline-Based Inks, Doping Agent Effect, and Advanced Electronic Devices Applications. Adv. Funct. Mater. 2014, 24, 3375–3383. [Google Scholar] [CrossRef]
- Diao, J.; Yuan, J.; Ding, A.; Zheng, J.; Lu, Z. Flexible Supercapacitor Based on Inkjet-Printed Graphene@Polyaniline Nanocomposites with Ultrahigh Capacitance. Macromol. Mater. Eng. 2018, 303, 1800092. [Google Scholar] [CrossRef]
- Xu, Y.; Hennig, I.; Freyberg, D.; James Strudwick, A.; Georg Schwab, M.; Weitz, T.; Chih-Pei Cha, K. Inkjet-Printed Energy Storage Device Using Graphene/Polyaniline Inks. J. Power Sources 2014, 248, 483–488. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Yang, J.; Wang, Y.; Chan-Park, M.B. Three-Dimensional Macroporous Graphene Foam Filled with Mesoporous Polyaniline Network for High Areal Capacitance. ACS Sustain. Chem. Eng. 2014, 2, 2291–2296. [Google Scholar] [CrossRef]
- Anjali, M.K.; Bharath, G.; Rashmi, H.M.; Avinash, J.; Naresh, K.; Raju, P.N.; Raghu, H.V. Polyaniline-Pectin Nanoparticles Immobilized Paper Based Colorimetric Sensor for Detection of Escherichia Coli in Milk and Milk Products. Curr. Res. Food Sci. 2022, 5, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Bilbao, E.; Kapadia, S.; Riechert, V.; Amalvy, J.; Molinari, F.N.; Escobar, M.M.; Baumann, R.R.; Monsalve, L.N. Functional Aqueous-Based Polyaniline Inkjet Inks for Fully Printed High-Performance PH-Sensitive Electrodes. Sens. Actuators B Chem. 2021, 346, 130558. [Google Scholar] [CrossRef]
- Crowley, K.; Morrin, A.; Hernandez, A.; O’Malley, E.; Whitten, P.G.; Wallace, G.G.; Smyth, M.R.; Killard, A.J. Fabrication of an Ammonia Gas Sensor Using Inkjet-Printed Polyaniline Nanoparticles. Talanta 2008, 77, 710–717. [Google Scholar] [CrossRef]
- Le, D.D.; Nguyen, T.N.N.; Doan, D.C.T.; Dang, T.M.D.; Dang, M.C. Fabrication of Interdigitated Electrodes by Inkjet Printing Technology for Apllication in Ammonia Sensing. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 025002. [Google Scholar] [CrossRef]
- Crowley, K.; Morrin, A.; Shepherd, R.L.; In Het Panhuis, M.; Wallace, G.G.; Smyth, M.R.; Killard, A.J. Fabrication of Polyaniline-Based Gas Sensors Using Piezoelectric Inkjet and Screen Printing for the Detection of Hydrogen Sulfide. IEEE Sens. J. 2010, 10, 1419–1426. [Google Scholar] [CrossRef]
- Sarfraz, J.; Tobjork, D.; Osterbacka, R.; Linden, M. Low-Cost Hydrogen Sulfide Gas Sensor on Paper Substrates: Fabrication and Demonstration. IEEE Sens. J. 2012, 12, 1973–1978. [Google Scholar] [CrossRef]
- Kit-Anan, W.; Olarnwanich, A.; Sriprachuabwong, C.; Karuwan, C.; Tuantranont, A.; Wisitsoraat, A.; Srituravanich, W.; Pimpin, A. Disposable Paper-Based Electrochemical Sensor Utilizing Inkjet-Printed Polyaniline Modified Screen-Printed Carbon Electrode for Ascorbic Acid Detection. J. Electroanal. Chem. 2012, 685, 72–78. [Google Scholar] [CrossRef]
- Kulkarni, M.V.; Apte, S.K.; Naik, S.D.; Ambekar, J.D.; Kale, B.B. Ink-Jet Printed Conducting Polyaniline Based Flexible Humidity Sensor. Sens. Actuators B Chem. 2013, 178, 140–143. [Google Scholar] [CrossRef]
- Li, L.; Pan, L.; Ma, Z.; Yan, K.; Cheng, W.; Shi, Y.; Yu, G. All Inkjet-Printed Amperometric Multiplexed Biosensors Based on Nanostructured Conductive Hydrogel Electrodes. Nano Lett. 2018, 18, 3322–3327. [Google Scholar] [CrossRef]
- Suman; O’Reilly, E.; Kelly, M.; Morrin, A.; Smyth, M.R.; Killard, A.J. Chronocoulometric Determination of Urea in Human Serum Using an Inkjet Printed Biosensor. Anal. Chim. Acta 2011, 697, 98–102. [Google Scholar] [CrossRef]
- Määttänen, A.; Vanamo, U.; Ihalainen, P.; Pulkkinen, P.; Tenhu, H.; Bobacka, J.; Peltonen, J. A Low-Cost Paper-Based Inkjet-Printed Platform for Electrochemical Analyses. Sens. Actuators B Chem. 2013, 177, 153–162. [Google Scholar] [CrossRef]
- Morrin, A.; Wilbeer, F.; Ngamna, O.; Moulton, S.E.; Killard, A.J.; Wallace, G.G.; Smyth, M.R. Novel Biosensor Fabrication Methodology Based on Processable Conducting Polyaniline Nanoparticles. Electrochem. Commun. 2005, 7, 317–322. [Google Scholar] [CrossRef]
- Oh, W.K.; Kim, S.; Shin, K.H.; Jang, Y.; Choi, M.; Jang, J. Inkjet-Printed Polyaniline Patterns for Exocytosed Molecule Detection from Live Cells. Talanta 2013, 105, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Tortorich, R.P.; Da Costa, T.H.; Choi, J.W. Inkjet Printing of Conductive Polymer Nanowire Network on Flexible Substrates and Its Application in Chemical Sensing. Microelectron. Eng. 2015, 145, 143–148. [Google Scholar] [CrossRef]
- Song, E.; da Costa, T.H.; Choi, J.W. A Chemiresistive Glucose Sensor Fabricated by Inkjet Printing. Microsyst. Technol. 2017, 23, 3505–3511. [Google Scholar] [CrossRef]
- Teengam, P.; Siangproh, W.; Tuantranont, A.; Henry, C.S.; Vilaivan, T.; Chailapakul, O. Electrochemical Paper-Based Peptide Nucleic Acid Biosensor for Detecting Human Papillomavirus. Anal. Chim. Acta 2017, 952, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, Y.; Chen, M.; Yao, H.; Zheng, X.; Yang, W. An Inkjet Printing Soft Photomask and Its Application on Organic Polymer Substrates. Sci. China Chem. 2010, 53, 1695–1704. [Google Scholar] [CrossRef]
- Zea, M.; Texidó, R.; Villa, R.; Borrós, S.; Gabriel, G. Specially Designed Polyaniline/Polypyrrole Ink for a Fully Printed Highly Sensitive PH Microsensor. Cite This ACS Appl. Mater. Interfaces 2021, 13, 33535. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, S.; Zhang, T.; Fisher, A.; Lee, J.Y. Al3+ Intercalation/de-Intercalation-Enabled Dual-Band Electrochromic Smart Windows with a High Optical Modulation, Quick Response and Long Cycle Life. Energy Environ. Sci. 2018, 11, 2884–2892. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, C.; Song, J.; Yun, Y.J.; Jun, Y.; Ah, C.S. Electrochromic Devices Based on Ultraviolet-Cured Poly(Methyl Methacrylate) Gel Electrolytes and Their Utilisation in Smart Window Applications. J. Mater. Chem. C 2020, 8, 8747–8754. [Google Scholar] [CrossRef]
- Cong, S.; Tian, Y.; Li, Q.; Zhao, Z.; Geng, F. Single-crystalline Tungsten Oxide Quantum Dots for Fast Pseudocapacitor and Electrochromic Applications. Adv. Mater. 2014, 26, 4260–4267. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Park, S.; Cheng, X.; Eh, A.L.S.; Lee, P.S. Inkjet-Printed Metal Oxide Nanoparticles on Elastomer for Strain-Adaptive Transmissive Electrochromic Energy Storage Systems. Sci. Technol. Adv. Mater. 2018, 19, 759–770. [Google Scholar] [CrossRef]
- Small, W.R.; Masdarolomoor, F.; Wallace, G.G.; In Het Panhuis, M. Inkjet Deposition and Characterization of Transparent Conducting Electroactive Polyaniline Composite Films with a High Carbon Nanotube Loading Fraction. J. Mater. Chem. 2007, 17, 4359–4361. [Google Scholar] [CrossRef]
- Shim, G.H.; Han, M.G.; Sharp-Norton, J.C.; Creager, S.E.; Foulger, S.H. Inkjet-Printed Electrochromic Devices Utilizing Polyaniline-Silica and Poly(3,4-Ethylenedioxythiophene)-Silica Colloidal Composite Particles. J. Mater. Chem. 2008, 18, 594–601. [Google Scholar] [CrossRef]
- Abargues, R.; Rodríguez-Cantó, P.J.; García-Calzada, R.; Martínez-Pastor, J. Patterning of Conducting Polymers Using UV Lithography: The in-Situ Polymerization Approach. J. Phys. Chem. C 2012, 116, 17547–17553. [Google Scholar] [CrossRef]
- Cho, J.; Shin, K.H.; Jang, J. Polyaniline Micropattern onto Flexible Substrate by Vapor Deposition Polymerization-Mediated Inkjet Printing. Thin Solid Film. 2010, 518, 5066–5070. [Google Scholar] [CrossRef]
- Jeon, S.; Park, S.; Nam, J.; Kang, Y.; Kim, J.M. Creating Patterned Conjugated Polymer Images Using Water-Compatible Reactive Inkjet Printing. ACS Appl. Mater. Interfaces 2016, 8, 1813–1818. [Google Scholar] [CrossRef]
- Rajzer, I.; Rom, M.; Menaszek, E.; Pasierb, P. Conductive PANI Patterns on Electrospun PCL/Gelatin Scaffolds Modified with Bioactive Particles for Bone Tissue Engineering. Mater. Lett. 2015, 138, 60–63. [Google Scholar] [CrossRef]
- Xu, Q.; Ihalainen, P.; Smått, J.H.; Määttänen, A.; Sund, P.; Wilén, C.E.; Peltonen, J. Template-Induced Fabrication of Nanopatterned Polymeric Films by Inkjet Printing. Appl. Surf. Sci. 2014, 313, 237–242. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Van, Q.L.; Peng, S.; Dai, Z.; Ahn, S.H.; Kim, S.Y. Exploring Conducting Polymers as a Promising Alternativefor Electrochromic Devices. Adv. Mater. Technol. 2023, 2300474. [Google Scholar] [CrossRef]










| Conductive Polymer | Ink Formulation and Device Fabrication | Applications | Highlights | Reference |
|---|---|---|---|---|
| Polyaniline | Reduction of GH-PANI/GOP by hydroiodic acid and simultaneously peeling off from the commercial paper substrate to give the freestanding electrode (GH-PANI/GP electrode). | Fabrication of graphene-based nanohybrid materials for use in many electronic systems. | A maximum energy density of 24.02 Wh kg−1 at a power density of 400.33 W kg−1 and a power density of 3202.4 W kg−1 at an energy density of 13.29 Wh kg−1 are achievable at an operating voltage of 0.8 V. | [57] |
| Polyaniline | Two distinct doped PANI inks were prepared by dissolving the emeraldine salt of PANI in dimethylsuphoxide, followed by the addition of trifluorosulphonic acid and camporsulphonic acid. | Materials with negative capacitance in the low-frequency range can be used in devices working in nominal grid conditions (50–60 Hz) and up to short-wave radio frequencies. | Negative capacitance is reported. The highest negative supercapacitance achieved is −2.3 mF at 30 Hz, corresponding to a specific mass capacity of −799 F g−1. | [58] |
| Polyaniline | Electrodes were fabricated by printing GO @ PANI composites on gold-coated polymer substrates and then reducing them. Sandwiched and interdigitated supercapacitors were developed. | This method allows the end users to precisely deposit active materials according to their designs for miniature and wearable electronics. | Devices fabricated have high volumetric capacities of 258.5 F cm−3 at 1 mV s−1 for sandwich structures and 554 F cm−3 at 1 mV s−1 for interdigitated ones. Even after 2000 cycles of charging and discharging, over 90% capacitance retention could be achieved. | [59] |
| Polyaniline | Graphene polyaniline (NGP/PANI) inks of appropriate surface tension and viscosity were formulated and then inkjet printed to produce thin-film supercapacitor electrodes. | This preparation method allowed good control over pattern geometry and location in thin films. A major application is manufacturing energy storage devices in printable electronics. | In 1 M H2SO4 solution as the electrolyte, a maximum specific capacitance of 82 Fg−1, a power density of 124 kW kg−1, and an energy density of 2.4 Wh kg−1 were observed when a scan rate of 20 mV s−1 was applied. A long life cycle of over 1000 cycles. | [60] |
| Polyaniline | Graphene foam is made up of a few layers of graphene electrodeposited with a thin layer of PANI, with subsequent filling of the submillimeter-sized pores with PANI by using inkjet printing. | Good option for high-performance supercapacitors. | The synergistic effect of graphene and PANI provides a large areal capacitance of over 1700 mF cm−2. | [61] |
| Conductive Polymer | Ink Formulation and Device Fabrication | Applications | Highlights | Reference |
|---|---|---|---|---|
| Polyaniline | PANI-PEC dispersion in ethanol was printed on Whatman filter paper, followed by UV sterilization | Qualitative and quantitative detection of E. coli in each solution | Incredible sensitivity of 0.52 ± 0.17 log CFU/mL Simple and cost-effective | [62] |
| Polyaniline | Acrylic resin-based inks were applied to SDS stabilized PANI lattices while maintaining a PANI: resin weight ratio of 1:1 (dry basis) | Compatible for analysis in wearable systems | Improved viscosity as well as wear resistance Stable electrodes with reproducible pH-sensing ability Great pH sensitivity (up to 69.1 mV/pH) | [63] |
| Polyaniline | Silver and carbon IDA’s were Screen printed while nano- PANI suspension was inkjet printed | Detection of ammonia in air | Thermally stable sensor with high sensitivity to gaseous ammonia Unaffected by moisture and volatile organic compounds Can be used at elevated temperatures Very responsive in the analytically important (1–100 ppm) range | [64] |
| Polyaniline | Inkjet-printed silver electrodes on Si/SiO2 were further drop-coated with a blend film of the emeraldine salt form of PANI and ethylene glycol (EG) | Detection of ammonia | Reliable output in the range of 0 to 100 ppm of ammonia gas Fast recovery time of about 15 min | [65] |
| Polyaniline | Alternate PANI and CuCl2 layers wereinkjet printed on silver and carbon interdigitated electrodes | Hydrogen-sulfide sensors for short-term analyses | High sensitivity to hydrogen sulfide of up to 2.5 ppmv (parts per million by volume) | [66] |
| Polyaniline | Inkjet-printed silver electrodes were drop-coated with dispersions of PANI/CuCl2 to form films | Food quality monitoring | Appreciable sensitivity of up to 10 ppm due to protonation of PANI by H2S Low-cost H2S gas detector and can be used for food quality monitoring The relatively low absolute resistance values allow for the switching on of an LED using a low-voltage battery in a simple sensor circuit | [67] |
| Polyaniline | Screen-printed carbon electrode (SPCE) was subjected to inkjet printing with PANI to form a working electrode | Efficient sensor for ascorbic acid | Good sensitivity of 17.7 μA/mM for ascorbic acid Low-cost, disposable, and point-of-care sensor | [68] |
| Polyaniline | The PANI ink was synthesized via oxidative polymerization | Low-cost RFID tags, polymer-based photovoltaic cells and in printed flexible electronic devices | Efficient at room temperature | [69] |
| Polyaniline | The substrate contains three carbon working electrodes and an Ag/AgCl shared counter and reference electrode PANI hydrogel was synthesized from phytic acid, ammonium persulfate, and aniline | The biosensor is efficient and multipurpose with capability of detecting glucose, lactates, and triglycerides with high accuracy Integrated multiplexed biosensors for monitoring of parameters in humans can be mass produced | Easy multiple-analyte detection by multiplexing multiple sensors on a chip The sensitivity with respect to triglycerides was found to be 7.49 μA/mM−1 cm−2 between 0.1 mM and 6 mM, while that of lactate was 3.94 μAmM−1 cm−2 between 0.08 mM and 5 mM The glucose sensitivity exhibited by the sensor was 5.03 μAmM−1 cm−2 between 1 mM and 25 mM | [70] |
| Polyaniline | Screen printing of carbon electrodes onto PET plates, followed by inkjet printing of PANI NPs, and urease enzyme solution on the working electrodes | Efficient urea detector in human serum samples | An efficient sensor to measure ammonia in the 0.1–100 mM range and urea in the 2–12 mM range (r2 = 0.98) | [71] |
| PEDOT, polyaniline | Inkjet-printed gold NPs function as the working and counter electrodes. Inkjet-printed silver nanoparticle electrode functions as the reference electrode | Good pH and glucose sensors | Reusable after rinsing the aqueous samples Sensitive to pH even after five weeks of storage Paper chip allows for fast and on-site analysis Cost-effective and easy Functions with low sample volumes | [72] |
| Polyaniline | PANI was used as NP, and the enzymes were drop-coated in parallel on the electrodes | Enzyme biosensing | Mass production is possible as no electrochemical processes are involved in fabrication | [73] |
| Polyaniline | Inkjet printing on PET films by deposition of PANI patterns and immobilization of RGD peptide over it by covalent linkages | Sensing biomolecules in live cells Neurotransmitter detection from live cells, tracking biomolecular release, and detection of exocytosed biomolecules | Good ability to translate and amplify exocytosis molecules into a detectable signals | [74] |
| Polyaniline | Inkjet printing of multiwalled carbon nanotube electrodes, followed by printing of randomly oriented PANI nanowires dispersed in an aqueous medium | pH and H2O2 sensor | 200-micrometer minimum printing resolution Point-of-care diagnostics | [75] |
| Polyanailine | Sequential inkjet printing of carbon nanotubes and polyaniline nanowires along with the glucose oxidase and platinum nanoparticle layers between the CNT layers | Excellent glucose sensor that has the potential to be an on-demand printable point-of-care diagnostic kit for glucose measurement | Quick and disposable Linear relationship between current measured and glucose concentration with a detection limit of 2 mM of glucose | [76] |
| Polyaniline | The AQ-PNA probe was immobilized on the working electrode, i.e., inkjet-printed G-PANI conductive ink onto the screen-printed carbon ink | A cost-effective sensor that can be incinerated for screening and monitoring of the amount of HPV-DNA type 16 to diagnose cervical cancer | A linear response range of 10–200 nM was obtained The detection limit of HPV type 16 DNA was found to be 2.3 nM Highly sensitive ePAD DNA biosensor | [77] |
| Biaxially oriented polypropylene covered with silica oxide (BOPP-SiO4) | The inkjet-printed soft photomasks were used for depositing organic polymers and inorganic materials on polymer films | Depositing organic polymers and inorganic material on polymer films Photografting organic polymers onto a polymer film Patterning on non-planar substrates | Utilized for making intricate patterns on non-planar substrates, microsensors, optical structures, and other devices that do not need to be extremely durable or dimensionally stable | [78] |
| Polyaniline, polypyrrole, and PSS | Inkjet printing of polyaniline, polypyrrole, and poly(sodium 4-styrenesulphonate) (PSS)-based inks deposited on gold microelectrode | Sensitive pH sensor that is stable over a wide pH range | Low-cost and disposable A linear super-Nernstian response (81.2 0.5 mV/pH unit) over a wide pH range (pH 3–10) is obtained | [79] |
| Conductive Polymer | Ink Composition and Device Fabrication | Applications | Highlights | Reference |
|---|---|---|---|---|
| WO3 nanoparticles | PEDOT:PSS buffer layer was spin coated onto the ST AgNW/PDMS followed by inkjet printing of the WO3 nanoparticle layer | Deformable and wearable electronics STEESDs with novel features | Large optical modulation of 40%, fast switching speed (<4.5 s), high coloration efficiency (75.5 cm2 C−1), and good stability and high specific capacity (32.3 mAh g−1 and 44.8 mAh cm−3) observed Good functionality and maintenance of electrochromic performance even when stretched up to 50–80% strain | [83] |
| Polyaniline | Water-soluble polyaniline composite materials with MWNT were dispersed in water and deposited via inkjet printing, yielding transparent conductive electroactive films | Development of inkjet printing as a viable tool for the fabrication of transparent conductive electroactive materials | These films allowed for the switching between yellow, green, and blue when printed onto photopaper, PET, Pt-ITO, and Au-PVDF substrates. A change in sheet resistance (1000–5000 ohm sq−1) and optical transmittance (30–70%) was reported with a change in the nanotube percentage | [84] |
| PANI–silica and PEDOT–silica | PANI–silica and PEDOT–silica composites converted via solvent exchange to intrinsically conductive-polymer inks, which were inkjet printed on indium-tin-oxide-coated poly (ethylene terephthalate) films | Electrochromic display device fabrication with various intrinsically conductive-polymer colloidal solutions | Color of the devices changed with change in potential. The color could also be tuned by inkjet-printed PANI–silica and PEDOT–silica blended particles as an electrochromic | [85] |
| Conductive Polymer | Ink Formulation and Device Fabrication | Applications | Highlights | Reference |
|---|---|---|---|---|
| Polyaniline | Vapor deposition polymerization (VDP)-mediated inkjet printing (VDP-IJP) The substrate undergoes chemical oxidation polymerization at elevated temperatures that form emeraldine salt PANI patterns | Micro-range accuracy Efficient PANI synthesis | Minimum width of patterned line = 80 μm Average sheet resistance = 3.8 × 103 Ωsq−1 Does not require surfactants or stabilizers | [87] |
| Poly(phenylenevinyelene) (PPV) | Reactive inkjet printing An in situ Wittig reaction results in the formation of PPV patterns | Readily generated PPV microarrays Patterning of functional organic materials on a solid substrate | High processability Easy removal of unreacted reagents and byproducts by dissolution in organic media | [88] |
| Polyaniline | Electrospinning to obtain a bilayer biodegradable scaffold for PANI printing | Bone tissue engineering | Stimulation of cellular functions like attachment, proliferation, migration, and differentiation | [89] |
| Polyaniline | Combination of nanotemplating and inkjet printing PBTL, and PANI-DNNSA inks were inkjet printed onto NP-TiO2 and ZFNP-TiO2 substrates | Biosensing and electronic devices | Low-cost and efficient | [90] |
| Polyaniline | Oxidative polymerization in an aqueous medium using polystyrene sulfonate (PSS) as an emulsioning or doping agent Controlling the amount of the oxidant resulted in mixed leucoemeraldine or emeraldine oxidation states | Ideal for devices where positive parasitic capacitances have to be compensated | Synthesis from the dimer DANI Negative capacitance Cost-effective and simple | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arora, E.K.; Sharma, V.; Ravi, A.; Shahi, A.; Jagtap, S.; Adhikari, A.; Dash, J.K.; Kumar, P.; Patel, R. Polyaniline-Based Ink for Inkjet Printing for Supercapacitors, Sensors, and Electrochromic Devices. Energies 2023, 16, 6716. https://doi.org/10.3390/en16186716
Arora EK, Sharma V, Ravi A, Shahi A, Jagtap S, Adhikari A, Dash JK, Kumar P, Patel R. Polyaniline-Based Ink for Inkjet Printing for Supercapacitors, Sensors, and Electrochromic Devices. Energies. 2023; 16(18):6716. https://doi.org/10.3390/en16186716
Chicago/Turabian StyleArora, Ekta Kundra, Vibha Sharma, Aravind Ravi, Akanksha Shahi, Shweta Jagtap, Arindam Adhikari, Jatis Kumar Dash, Pawan Kumar, and Rajkumar Patel. 2023. "Polyaniline-Based Ink for Inkjet Printing for Supercapacitors, Sensors, and Electrochromic Devices" Energies 16, no. 18: 6716. https://doi.org/10.3390/en16186716