Gasification Characteristics of High Moisture Content Lignite under CO2 and Auto-Generated Steam Atmosphere in a Moving Bed Tubular Reactor
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Preparation of Lignite Samples
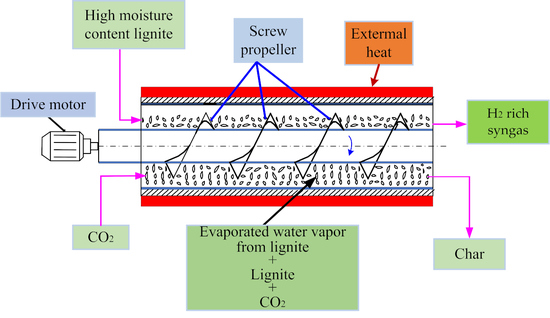
2.2. Experimental Instruments and Procedure
2.3. Analysis of the Gasification Product
3. Results and Discussion
3.1. Brief Description of the Gasification Process
3.2. Effect of Temperature
3.3. Effect of CO2 Flow Rate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shan, Y.; Guan, D.; Hubacek, K.; Zheng, B.; Davis, S.J.; Jia, L.; Liu, J.; Liu, Z.; Fromer, N.; Mi, Z.; et al. City-level climate change mitigation in China. Sci. Adv. 2018, 207, 582–589. [Google Scholar] [CrossRef]
- He, X.; Zeng, K.; Xie, Y.; Flamant, G.; Yang, H.; Yang, X.; Nzihou, A.; Zheng, A.; Ding, Z.; Chen, H. The effects of temperature and molten salt on solar pyrolysis of lignite. Energy 2019, 181, 407–416. [Google Scholar] [CrossRef]
- Liu, R.; Liu, M.; Fan, P.; Zhao, Y.; Yan, J. Thermodynamic study on a novel lignite poly-generation system of electricity-gas-tar integrated with pre-drying and pyrolysis. Energy 2018, 165, 140–152. [Google Scholar] [CrossRef]
- Zhou, G.; Huang, Q.; Yu, B.; Tong, H.; Chi, Y.; Yan, J. Effect of industrial microwave irradiation on the physicochemical properties and pyrolysis characteristics of lignite. Chin. J. Chem. Eng. 2018, 26, 1171–1178. [Google Scholar] [CrossRef]
- Haus, J.; Goltzsche, M.; Hartge, E.-U.; Heinrich, S.; Werther, J. Gasification kinetics of lignite char in a fluidized bed of reactive oxygen carrier particles. Fuel 2019, 236, 166–178. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, G.; Reinmöller, M.; Meyer, B. Effect of inherent mineral matter on the co-pyrolysis of highly reactive brown coal and wheat straw. Fuel 2019, 239, 1194–1203. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Song, Q.; Liu, S.; Yan, J.; Wang, X.; Ma, Q.; Shu, X. Investigation on the physicochemical structure and gasification reactivity of nascent pyrolysis and gasification char prepared in the entrained flow reactor. Fuel 2019, 240, 126–137. [Google Scholar] [CrossRef]
- Zhao, K.; Fang, X.; Huang, Z.; Wei, G.; Zheng, A.; Zhao, Z. Hydrogen-rich syngas production from chemical looping gasification of lignite by using NiFe2O4 and CuFe2O4 as oxygen carriers. Fuel 2021, 303, 121269. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Ban, Y.; Song, Y.; Zhi, K.; Teng, Y.; He, R.; Zhou, H.; Liu, Q.; Qi, Y. Direct production of high hydrogen syngas by steam gasification of Shengli lignite/chars: Remarkable promotion effect of inherent minerals and pyrolysis temperature. Int. J. Hydrogen Energy 2017, 42, 5865–5872. [Google Scholar] [CrossRef]
- Li, C.-Z. Some recent advances in the understanding of the pyrolysis and gasification behaviour of Victorian brown coal. Fuel 2007, 86, 1664–1683. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, S.; Tursun, Y.; Wang, C.; Wang, G. Catalytic steam gasification of lignite for hydrogen-rich gas production in a decoupled triple bed reaction system. Fuel 2017, 189, 57–65. [Google Scholar] [CrossRef]
- Özdenkçi, K.; Prestipino, M.; Björklund-Sänkiaho, M.; Galvagno, A.; De Blasio, C. Alternative energy valorization routes of black liquor by stepwise supercritical water gasification: Effect of process parameters on hydrogen yield and energy efficiency. Renew. Sustain. Energy Rev. 2020, 134, 110146. [Google Scholar] [CrossRef]
- Liu, J.; Hu, N.; Fan, L.-W. Optimal design and thermodynamic analysis on the hydrogen oxidation reactor in a combined hydrogen production and power generation system based on coal gasification in supercritical water. Energy 2022, 238, 121862. [Google Scholar] [CrossRef]
- Adar, E.; Ince, M.; Bilgili, M.S. Supercritical water gasification of sewage sludge by continuous flow tubular reactor: A pilot scale study. Chem. Eng. J. 2020, 391, 123499. [Google Scholar] [CrossRef]
- Gao, H.; Zhu, Y.; Fu, F.; Wu, H.; Du, Y.; Chen, H.; Liao, C.; Fan, H. Pyrolysis of Hailar lignite in an autogenerated steam agent. J. Therm. Anal. Calorim. 2014, 117, 973–978. [Google Scholar] [CrossRef]
- Li, G.-Y.; Li, A.-Q.; Zhang, H.; Wang, J.-P.; Chen, S.-Y.; Liang, Y.-H. Theoretical study of the CO formation mechanism in the CO2 gasification of lignite. Fuel 2018, 211, 353–362. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Ding, J.-X.; Li, G.-Y.; Wang, J.-P.; Tian, Y.; Liang, Y.-H. Theoretical study of the formation mechanism of sulfur-containing gases in the CO2 gasification of lignite. Fuel 2019, 242, 398–407. [Google Scholar] [CrossRef]
- Kumari, G.; Vairakannu, P. CO2-air based two stage gasification of low ash and high ash Indian coals in the context of underground coal gasification. Energy 2018, 143, 822–832. [Google Scholar] [CrossRef]
- Sripada, P.P.; Xu, T.; Kibria, M.A.; Bhattacharya, S. Comparison of entrained flow gasification behaviour of Victorian brown coal and biomass. Fuel 2017, 203, 942–953. [Google Scholar] [CrossRef]
- Tanner, J.; Bhattacharya, S.; Bläsing, M.; Müller, M. High-temperature pyrolysis and CO2 gasification of Victorian brown coal and Rhenish lignite in an entrained flow reactor. AIChE J. 2016, 62, 2101–2111. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, Y.; Yang, L.; Zhu, Y. High quality syngas produced from the co-pyrolysis of wet sewage sludge with sawdust. Int. J. Hydrogen Energy 2018, 43, 5463–5472. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, H.; Guo, X.; Gong, X. Effect of water addition on flow properties of lignite particles. Chem. Eng. Res. Des. 2018, 132, 1020–1029. [Google Scholar] [CrossRef]
- Artok, L.; Schobert, H.H.; Nomura, M.; Erbatur, O.; Kidena, K. Effects of water and molecular hydrogen on heat treatment of Turkish low-rank coals. Energy Fuels 1998, 12, 1200–1211. [Google Scholar] [CrossRef]
- Li, C.; Liao, J.-J.; Yin, Y.; Mo, Q.; Chang, L.-P.; Bao, W.-R. Kinetic analysis on the microwave drying of different forms of water in lignite. Fuel Process. Technol. 2018, 176, 174–181. [Google Scholar] [CrossRef]
- Xiong, S.; Zhuo, J.; Zhang, B.; Yao, Q. Effect of moisture content on the characterization of products from the pyrolysis of sewage sludge. J. Anal. Appl. Pyrolysis 2013, 104, 632–639. [Google Scholar] [CrossRef]
- Wang, J.H.; Li, F.; Chang, L.P.; Xie, K.-C. The Structure Characteristics and Reactivity of Lingwu Coal and Its Macerals in Western China. Energy Sources Part A Recovery Util. Environ. Eff. 2010, 32, 1869–1877. [Google Scholar] [CrossRef]
- Roberts, D.G.; Harris, D.J. Char gasification in mixtures of CO2 and H2O: Competition and inhibition. Fuel 2007, 86, 2672–2678. [Google Scholar] [CrossRef]
- Tong, S.; Li, L.; Duan, L.; Zhao, C.; Anthony, E.J. A kinetic study on lignite char gasification with CO2 and H2O in a fluidized bed reactor. Appl. Therm. Eng. 2019, 147, 602–609. [Google Scholar] [CrossRef]
- Samih, S.; Chaouki, J. Catalytic ash free coal gasification in a fluidized bed thermogravimetric analyzer. Powder Technol. 2017, 316, 551–559. [Google Scholar] [CrossRef]
- Xu, T.; Pisupati, S.V.; Bhattacharya, S. Comparison of entrained flow CO2 gasification behaviour of three low-rank coals—Victorian brown coal, Beulah lignite, and Inner Mongolia lignite. Fuel 2019, 249, 206–218. [Google Scholar] [CrossRef]
- Saucedo, M.A.; Lim, J.Y.; Dennis, J.S.; Scott, S.A. CO2-gasification of a lignite coal in the presence of an iron-based oxygen carrier for chemical-looping combustion. Fuel 2014, 127, 186–201. [Google Scholar] [CrossRef]
- Tanner, J.; Bhattacharya, S. Kinetics of CO2 and steam gasification of Victorian brown coal chars. Chem. Eng. J. 2016, 285, 331–340. [Google Scholar] [CrossRef]
- Lee, R.; Sohn, J.M. A study on the effect of the CO2/steam mixtures and the addition of natural minerals on the reactivity of Adaro coal gasification. Int. J. Hydrogen Energy, 2022. (in press) [Google Scholar]
- Gül, S.; Akgün, F.; Aydar, E.; Ünlü, N. Pressurized gasification of lignite in a pilot scale bubbling fluidized bed reactor with air, oxygen, steam and CO2 agents. Appl. Therm. Eng. 2018, 130, 203–210. [Google Scholar] [CrossRef]






| Proximate Analysis (wt.%, ar) | Ultimate Analysis (wt.%, daf) | |||||||
|---|---|---|---|---|---|---|---|---|
| M | A | V | FC | C | H | O | S | N |
| 30.41 | 15.26 | 30.97 | 23.36 | 79.81 | 4.17 | 14.33 | 0.56 | 1.13 |
| System Parameter | Range/Set Point |
|---|---|
| Lignite feed rate | 10–30 g min−1 |
| Lignite particle size | 0.5–3.0 mm−1 |
| Residence time | 20 min |
| Isothermal temperature of the spiral propelled reactor | 800–1100 °C |
| Reactor hot zone length | 1200 mm |
| Reactor diameter | 50 mm |
| Volume of the spiral propelled reactor | 2.3 L |
| System pressure | 50 kPa |
| Temperature /°C | Volume Fraction/% | H2/CO | QLHV /(MJ Nm−3) | Gas Yield/mol kg−1 | Carbon Conversion Ratio/% | ||||
|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CH4 | CO2 | C2Hn | |||||
| 800 | 39.92 | 25.08 | 9.17 | 24.47 | 1.36 | 1.59 | 11.62 | 21.17 | 25.75 |
| 850 | 41.23 | 26.14 | 8.92 | 22.36 | 1.35 | 1.58 | 11.80 | 24.69 | 29.39 |
| 900 | 43.72 | 29.81 | 8.12 | 17.04 | 1.31 | 1.47 | 12.22 | 29.88 | 34.07 |
| 950 | 43.98 | 31.62 | 6.67 | 16.81 | 0.92 | 1.39 | 11.71 | 34.95 | 39.39 |
| 1000 | 45.08 | 34.64 | 5.04 | 14.63 | 0.61 | 1.30 | 11.43 | 39.62 | 43.55 |
| Flow Rate/(L min−1) | Volume Fraction/% | H2/CO | QLHV/(MJ Nm−3) | Gas Yield/mol kg−1 | Carbon Conversion Ratio/% | ||||
|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CH4 | CO2 | C2Hn | |||||
| 0 | 51.72 | 24.13 | 7.90 | 14.41 | 1.84 | 2.14 | 12.62 | 18.86 | 18.71 |
| 0.8 | 47.45 | 27.04 | 8.06 | 15.32 | 2.13 | 1.75 | 12.77 | 25.33 | 27.41 |
| 1.1 | 43.72 | 29.81 | 8.12 | 17.04 | 1.31 | 1.47 | 12.22 | 29.88 | 34.07 |
| 1.5 | 35.68 | 34.92 | 8.87 | 19.61 | 0.92 | 1.02 | 12.02 | 34.45 | 44.49 |
| 2.0 | 31.15 | 37.56 | 7.74 | 22.86 | 0.69 | 0.81 | 11.73 | 40.09 | 55.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Wen, Z.; Jin, L.; Xiong, X.; Zhu, Y. Gasification Characteristics of High Moisture Content Lignite under CO2 and Auto-Generated Steam Atmosphere in a Moving Bed Tubular Reactor. Energies 2022, 15, 6751. https://doi.org/10.3390/en15186751
Gao H, Wen Z, Jin L, Xiong X, Zhu Y. Gasification Characteristics of High Moisture Content Lignite under CO2 and Auto-Generated Steam Atmosphere in a Moving Bed Tubular Reactor. Energies. 2022; 15(18):6751. https://doi.org/10.3390/en15186751
Chicago/Turabian StyleGao, Haojie, Zhisong Wen, Lizhu Jin, Xin Xiong, and Yuezhao Zhu. 2022. "Gasification Characteristics of High Moisture Content Lignite under CO2 and Auto-Generated Steam Atmosphere in a Moving Bed Tubular Reactor" Energies 15, no. 18: 6751. https://doi.org/10.3390/en15186751