Biodiesel Production through Acid Catalyst In Situ Reactive Extraction of Chlorella vulgaris Foamate
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
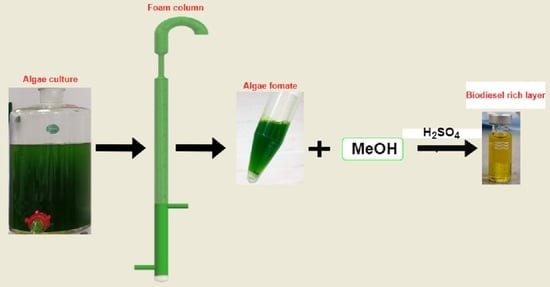
2.2. Experimental Procedure
2.3. Analytical Methods
2.3.1. Moisture Content Measurement
2.3.2. Chlorella vulgaris Microalgae Total Maximum Fames and Total Lipid
2.3.3. Determination of Total Mass of Methyl Ester by Gas Chromatography with Flame Ionization Detector
3. Results and Discussion
3.1. Process Parameters
3.1.1. Methanol/Oil Mole Ratio and Reaction Temperature
3.1.2. Reaction Time
3.2. Kinetic Study of the Transesterification Process
3.3. Determination of the Activation Energy
3.4. Thermodynamic Parameters for Microalgae Biodiesel Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, G.; Kumar, D.; Singh, S.; Kothari, S.; Bhatt, S.; Singh, C. Continuous Low Cost Transesterification Process for the Production of Coconut Biodiesel. Energies 2010, 3, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Santori, G.; di Nicola, G.; Moglie, M.; Polonara, F. A review analyzing the industrial biodiesel production practice starting from vegetable oil refining. Appl. Energy 2012, 92, 109–132. [Google Scholar] [CrossRef] [Green Version]
- Ziolkowska, J.R. Prospective technologies, feedstocks and market innovations for ethanol and biodiesel production in the US. Biotechnol. Rep. 2014, 4, 94–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, K.; Mahajan, D.; Kerr, R.; da Silva, M. Global Biofuels at the Crossroads: An Overview of Technical, Policy, and Investment Complexities in the Sustainability of Biofuel Development. Agriculture 2017, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Faruque, M.O.; Razzak, S.A.; Hossain, M.M. Application of Heterogeneous Catalysts for Biodiesel Production from Microalgal Oil—A Review. Catalysts 2020, 10, 1025. [Google Scholar] [CrossRef]
- Demirbaş, A. Production of Biodiesel from Algae Oils. Energy Sources Part A Recovery Util. Environ. Eff. 2008, 31, 163–168. [Google Scholar] [CrossRef]
- Balat, M. Production of Biodiesel from Vegetable Oils: A Survey. Energy Sources Part A Recovery Util. Environ. Eff. 2007, 29, 895–913. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Xia, L.; Li, Y.; Huang, R.; Song, S. Effective harvesting of microalgae by coagulation-flotation. R. Soc. Open Sci. 2017, 4, 170867. [Google Scholar] [CrossRef] [Green Version]
- Laamanen, C.A.; Ross, G.M.; Scott, J.A. Flotation harvesting of microalgae. Renew. Sustain. Energy Rev. 2016, 58, 75–86. [Google Scholar] [CrossRef]
- Bilad, M.R.; Arafat, H.A.; Vankelecom, I.F.J. Membrane technology in microalgae cultivation and harvesting: A review. Biotechnol. Adv. 2014, 32, 1283–1300. [Google Scholar] [CrossRef] [PubMed]
- Coward, T.; Lee, J.G.M.; Caldwell, G.S. Development of a foam flotation system for harvesting microalgae biomass. Algal Res. 2013, 2, 135–144. [Google Scholar] [CrossRef]
- Delrue, F.; Setier, P.A.; Sahut, C.; Cournac, L.; Roubaud, A.; Peltier, G.; Froment, A.K. An economic, sustainability, and energetic model of biodiesel production from microalgae. Bioresour. Technol. 2012, 111, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Alkarawi, M.A.S.; Caldwell, G.S.; Lee, J.G.M. Continuous harvesting of microalgae biomass using foam flotation. Algal Res. 2018, 36, 125–138. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Biodiesel production through sulfuric acid catalyzed transesterification of acidic oil: Techno economic feasibility of different process alternatives. Energy Convers. Manag. 2018, 174, 639–648. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, H.; Lin, L. Biodiesel: An Alternative to Conventional Fuel. Energy Procedia 2012, 16, 1874–1885. [Google Scholar] [CrossRef] [Green Version]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Chong, W.T.; Lam, M.K.; Loh, P.K.; Vellayan, V. Microalgae biofuels as an alternative to fossil fuel for power generation. Renew. Sustain. Energy Rev. 2016, 58, 180–197. [Google Scholar] [CrossRef]
- Aliyu, A.; Lee, J.G.M.; Harvey, A.P. Microalgae for biofuels via thermochemical conversion processes: A review of cultivation, harvesting and drying processes, and the associated opportunities for integrated production. Bioresour. Technol. Rep. 2021, 14, 100676. [Google Scholar] [CrossRef]
- Gunerken, E.; D’Hondt, R.; Eppink, M.H.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef]
- Kim, D.Y.; Vijayan, D.; Praveenkumar, R.; Han, J.I.; Lee, K.; Park, J.Y.; Chang, W.S.; Lee, J.S.; Oh, Y.K. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Byreddy, A.R.; Gupta, A.; Barrow, C.J.; Puri, M. Comparison of Cell Disruption Methods for Improving Lipid Extraction from Thraustochytrid Strains. Mar. Drugs 2015, 13, 5111–5127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Yuan, W.; Jiang, X.; Jing, Y.; Wang, Z. Disruption of microalgal cells using high-frequency focused ultrasound. Bioresour. Technol. 2014, 153, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Hattab, M.A. Microalgae Harvesting Methods for Industrial Production of Biodiesel: Critical Review and Comparative Analysis. J. Fundam. Renew. Energy Appl. 2015, 5, 1000154. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.C.; Kim, J.D. Cationic surfactant-based method for simultaneous harvesting and cell disruption of a microalgal biomass. Bioresour. Technol. 2013, 149, 579–581. [Google Scholar] [CrossRef]
- Lai, Y.S.; Francesco, F.D.; Aguinaga, A.; Parameswaran, P.; Rittmann, B.E. Improving lipid recovery from Scenedesmus wet biomass by surfactant-assisted disruption. Green Chem. 2016, 18, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Thimmaraju, R.; Bhagyalakshmi, N.; Narayan, M.S.; Ravishankar, G.A. Food-grade chemical and biological agents permeabilize red beet hairy roots, assisting the release of betalaines. Biotechnol. Prog. 2003, 19, 1274–1282. [Google Scholar] [CrossRef]
- Rupprecht, H.; Gu, T. Structure of adsorption layers of ionic surfactants at the solid/liquid interface. Colloid Polym. Sci. 1991, 269, 506–522. [Google Scholar] [CrossRef]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef]
- Brown, R.B.; Audet, J. Current techniques for single-cell lysis. J. R. Soc. Interface 2008, 5 (Suppl. S2), S131–S138. [Google Scholar] [CrossRef] [Green Version]
- Ulloa, G.; Coutens, C.; Sánchez, M.; Sineiro, J.; Fábregas, J.; Deive, F.J.; Rodríguez, A.; Núñez, M.J. On the double role of surfactants as microalga cell lysis agents and antioxidants extractants. Green Chem. 2012, 14, 1044–1051. [Google Scholar] [CrossRef]
- Nasirpour, N.; Mousavi, S.M.; Shojaosadati, S.A. A novel surfactant-assisted ionic liquid pretreatment of sugarcane bagasse for enhanced enzymatic hydrolysis. Bioresour. Technol. 2014, 169, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Coward, T.; Lee, J.G.M.; Caldwell, G.S. Harvesting microalgae by CTAB-aided foam flotation increases lipid recovery and improves fatty acid methyl ester characteristics. Biomass Bioenergy 2014, 67, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Hewson, J.C.; Amendola, P.; Reynoso, M.; Sommerfeld, M.; Chen, Y.; Hu, Q. Critical evaluation and modeling of algal harvesting using dissolved air flotation. Biotechnol. Bioeng. 2014, 111, 2477–2485. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Sharma, M.P. Review of process parameters for biodiesel production from different feedstocks. Renew. Sustain. Energy Rev. 2016, 62, 1063–1071. [Google Scholar] [CrossRef]
- Li, X.; Evans, G.M.; Stevenson, P. Process intensification of foam fractionation by successive contraction and expansion. Chem. Eng. Res. Des. 2011, 89, 2298–2308. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Evans, G.M.; Stevenson, P. Foam flowing vertically upwards in pipes through expansions and contractions. Int. J. Multiph. Flow 2011, 37, 802–811. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- EN 14103; Fat and Oil Derivatives-Fatty Acid Methyl Esters (FAME)—Determination of Ester and Linolenic Acid Methyl Ester Contents. European Committee for Standardization: Brussels, Belgium, 2003.
- Landels, A.; Beacham, T.A.; Evans, C.T.; Carnovale, G.; Raikova, S.; Cole, I.S.; Goddard, P.; Chuck, C.; Allen, M.J. Improving electrocoagulation floatation for harvesting microalgae. Algal Res. 2019, 39, 101446. [Google Scholar] [CrossRef]
- Hasni, K.; Ilham, Z.; Dharma, S.; Varman, M. Optimization of biodiesel production from Brucea javanica seeds oil as novel non-edible feedstock using response surface methodology. Energy Convers. Manag. 2017, 149, 392–400. [Google Scholar] [CrossRef]
- Baskar, G.; Gurugulladevi, A.; Nishanthini, T.; Aiswarya, R.; Tamilarasan, K. Optimization and kinetics of biodiesel production from Mahua oil using manganese doped zinc oxide nanocatalyst. Renew. Energy 2017, 103, 641–646. [Google Scholar] [CrossRef]
- Nautiyal, P.; Subramanian, K.; Dastidar, M. Kinetic and thermodynamic studies on biodiesel production from Spirulina platensis algae biomass using single stage extraction-transesterification process. Fuel 2014, 135, 228–234. [Google Scholar] [CrossRef]
- Nelson, D.R.; Viamajala, S. One-pot synthesis and recovery of fatty acid methyl esters (FAMEs) from microalgae biomass. Catal. Today 2016, 269, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, R.; Harvey, A.P. Direct production of biodiesel from rapeseed by reactive extraction/in situ transesterification. Fuel Process. Technol. 2012, 102, 53–60. [Google Scholar] [CrossRef]
- Salam, K.A.; Velasquez-Orta, S.; Harvey, A.P. A sustainable integrated in situ transesterification of microalgae for biodiesel production and associated co-product—A review. Renew. Sustain. Energy Rev. 2016, 65, 1179–1198. [Google Scholar] [CrossRef]
- Vicente, G.; Bautista, L.F.; Rodríguez, R.; Gutiérrez, F.J.; Sádaba, I.; Ruiz-Vázquez, R.M.; Torres-Martínez, S.; Garre, V. Biodiesel production from biomass of an oleaginous fungus. Biochem. Eng. J. 2009, 48, 22–27. [Google Scholar] [CrossRef]
- Viêgas, C.V.; Hachemi, I.; Freitas, S.P.; Mäki-Arvela, P.; Aho, A.; Hemming, J.; Smeds, A.; Heinmaa, I.; Fontes, F.B.; da Silva Pereira, D.C.; et al. A route to produce renewable diesel from algae: Synthesis and characterization of biodiesel via in situ transesterification of Chlorella alga and its catalytic deoxygenation to renewable diesel. Fuel 2015, 155, 144–154. [Google Scholar] [CrossRef]
- Velasquez-Orta, S.B.; Lee, J.G.M.; Harvey, A. Alkaline in situ transesterification of Chlorella vulgaris. Fuel 2012, 94, 544–550. [Google Scholar] [CrossRef]
- Haas, M.J.; Wagner, K. Simplifying biodiesel production: The direct or in situ transesterification of algal biomass. Eur. J. Lipid Sci. Technol. 2011, 113, 1219–1229. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, Z. Biodiesel production by direct methanolysis of oleaginous microbial biomass. J. Chem. Technol. Biotechnol. 2007, 82, 775–780. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Lam, M.K.; Uemura, Y.; Mansor, N.; Lim, J.W.; Show, P.L.; Tan, I.S.; Lim, S. High biodiesel yield from wet microalgae paste via in-situ transesterification: Effect of reaction parameters towards the selectivity of fatty acid esters. Fuel 2020, 272, 117718. [Google Scholar] [CrossRef]
- Sitepu, E.K.; Corbin, K.; Luo, X.; Pye, S.J.; Tang, Y.; Leterme, S.C.; Heimann, K.; Raston, C.L.; Zhang, W. Vortex fluidic mediated direct transesterification of wet microalgae biomass to biodiesel. Bioresour. Technol. 2018, 266, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Chamola, R.; Khan, M.F.; Raj, A.; Verma, M.; Jain, S. Response surface methodology based optimization of in situ transesterification of dry algae with methanol, H2SO4 and NaOH. Fuel 2019, 239, 511–520. [Google Scholar] [CrossRef]
- Feyzi, M.; Shahbazi, Z. Preparation, kinetic and thermodynamic studies of Al-Sr nanocatalysts for biodiesel production. J. Taiwan Inst. Chem. Eng. 2017, 71, 145–155. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Alagumalai, A.; Mathimani, T.; Atabani, A.E. Optimization, kinetic and thermodynamic studies on sustainable biodiesel production from waste cooking oil: An Indian perspective. Fuel 2020, 273, 117725. [Google Scholar] [CrossRef]
- Roy, T.; Sahani, S.; Chandra Sharma, Y. Study on kinetics-thermodynamics and environmental parameter of biodiesel production from waste cooking oil and castor oil using potassium modified ceria oxide catalyst. J. Clean. Prod. 2020, 247, 119166. [Google Scholar] [CrossRef]










| Feedstock | Temperature (°C) | Solvent | Catalyst (Oil Basis) (mol/mol) | Molar Ratio (Solvent: Oil) | Reaction Time (min) | Max. Yield (Oil Basis) (%) | References |
|---|---|---|---|---|---|---|---|
| Chlorella vulgaris | 70 | MeOH | H2SO4 0.85:1 | 1000:1 | 90 | 96 | This study |
| Chlorella vulgaris | 60 | MeOH | H2SO4 650:1 | 10,000:1 | 120 | ca.96 | [52] |
| Chloroparva pannonica | 25 | MeOH | H2SO4 0.70:1 | 830:1 | 15 | ca.97 | [53] |
| Dry Chlorella vulgaris | 50 | MeOH | H2SO4 0.336:1 | 800:1 | 60 | ca. 90 | [54] |
| Chlorella vulgaris | 60 | MeOH | H2SO4 0.35:1 | 600:1 | 1200 | 97 | [49] |
| Parameter (Unit) | Value |
|---|---|
| Chlorella vulgaris microalgae mass (g) | 0.8 |
| Oil content (%) | 48 |
| Mass of oil (g) | 0.384 |
| Molecular weight of oil (g/mole) | 880 |
| No. mole of oil (mole) Density of algae (g/mL) Volume of algae (mL) Catalyst type Catalyst/oil molar ratio No. mole of catalyst (mole) Molecular weight of catalyst (g/mole) Mass of H2SO4 (g) Density of catalyst (g/mL) Volume of catalyst (mL) Methannol/oil molar ratio No. mole of methanol (mole) Molecular weight of methanol (g/mole) Mass of methanol (g) Density of methanol (g/mL) Volume of methanol (mL) Total volume of reaction mixture (mL) Initial oil concentration (mole/L) Initial methanol concentration (mole/L) Initial catalyst concentration (mole/L) | 0.00044 0.896 0.9 H2SO4 8.5 0.0037 98.1 0.36 1.84 0.2 700 0.308 32.04 9.86 0.791 12.5 13.6 0.032 24.6 0.27 |
| MeOH/Oil Mole Ratio | Temperature (°C) | Apparent Pseudo First Order Forward Rate Constants, (min−1) | Actual Pseudo First Order Forward Rate Constants, kf (min−1) | Pseudo Second Order Backward Rate Constants, kb (L/mol.min) |
|---|---|---|---|---|
| 100 | 30 | 0.0096 | 0.00049 | 0.0349 |
| 50 | 0.0185 | 0.00095 | 0.0291 | |
| 70 | 0.0241 | 0.0012 | 0.0230 | |
| 400 | 30 | 0.0203 | 0.0021 | 0.0887 |
| 50 | 0.0383 | 0.0039 | 0.0585 | |
| 70 | 0.0493 | 0.0051 | 0.0428 | |
| 700 | 30 | 0.0305 | 0.0049 | 0.1098 |
| 50 | 0.0569 | 0.0093 | 0.0629 | |
| 70 | 0.0751 | 0.0123 | 0.0323 | |
| 1000 | 30 | 0.0344 | 0.0073 | 0.1386 |
| 50 | 0.0656 | 0.0140 | 0.0678 | |
| 70 | 0.0867 | 0.0186 | 0.0304 |
| Parameter | Value | Unit |
|---|---|---|
| Pre-exponential factor (A) | 51.4 | min−1 |
| activation energy (Ea) | 18.7 | kJ/mol |
| Thermodynamic Properties | Value | Unit |
|---|---|---|
| ΔH | 16.63 | kJ/mol |
| ΔS | −219.1 | J/mol.K |
| T (K) | ΔG (kJ/mol) |
|---|---|
| 303.15 | 83.0 |
| 323.15 | 87.4 |
| 343.15 | 91.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Humairi, S.T.; Lee, J.G.M.; Salihu, M.; Harvey, A.P. Biodiesel Production through Acid Catalyst In Situ Reactive Extraction of Chlorella vulgaris Foamate. Energies 2022, 15, 4482. https://doi.org/10.3390/en15124482
Al-Humairi ST, Lee JGM, Salihu M, Harvey AP. Biodiesel Production through Acid Catalyst In Situ Reactive Extraction of Chlorella vulgaris Foamate. Energies. 2022; 15(12):4482. https://doi.org/10.3390/en15124482
Chicago/Turabian StyleAl-Humairi, Shurooq T., Jonathan G. M. Lee, Musa Salihu, and Adam P. Harvey. 2022. "Biodiesel Production through Acid Catalyst In Situ Reactive Extraction of Chlorella vulgaris Foamate" Energies 15, no. 12: 4482. https://doi.org/10.3390/en15124482