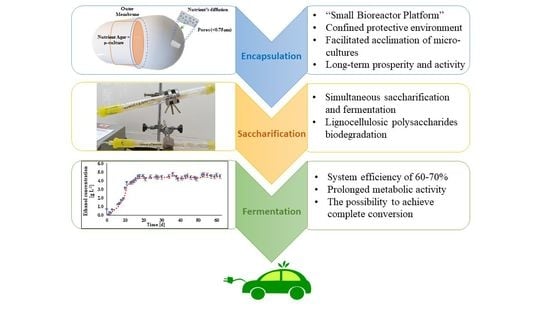
1. Introduction
In recent decades, global energy demand has continued to grow due to the fact of strong dependence on fossil fuels, a rapidly expanding world population, and increased industrial prosperity. These factors—combined with diminishing petroleum resources, intensive consumption of petroleum derivatives, and greenhouse gas emissions (e.g., CO
2 and CH
4)—have led to environmental degradation and political conflict [
1,
2,
3]. To date, the conventional rate of fossil fuel production has failed to keep pace with increasing world demand. To reduce the utilization of fossil energy sources and mitigate climate change, alternative methods for renewable green energy production are necessary [
1,
4].
A biorefinery can be seen as analogous to an oil refinery. However, in biorefinery the raw materials are natural renewable biomasses. Thus, a biorefinery holds promising potential through close-to-uniform feedstock and conversion through inexpensive and simple processes [
5,
6]. Produced biofuels are classified as 1st, 2nd, and 3rd generation biorefinery technologies according to the physical and chemical nature of the raw materials. First-generation biorefinery technology is based on seeds and grains (e.g., sugar, starch, and corn). This approach is not considered sustainable due to the differing crop availability only in certain seasons, spontaneous degradation, consumption of food crops, and use of water resources and fertile soils [
6]. Second-generation technology is based on nonedible crops such as lignocellulosic products (e.g., wood and grass), industry and forestry residues, and side or waste streams (e.g., black liquor, straw, and sawdust) [
1,
6,
7]. The distinct advantages of the 2nd generation are the abundance of feedstock that is independent of the seasons, its constantly replaceable nature, and the ack of competition with food consumption [
1,
8]. The main disadvantages of this method are costly pre-treatment of the biomass and complex processes of converting raw materials into fuel [
9]. Third-generation biorefinery is based on algae crops (or microalgae) due to their rapid growth, and increased photosynthesis, and ability to convert CO
2 into higher-value products [
9,
10]. This research field is novel and suffers from several disadvantages such as high amounts of CO
2 required, expensive commercial scale-up, unresolved contamination issues, and environmental, and social issues [
11].
Lignocellulose is a complex substrate composed of three main components: cellulose, hemicellulose, and lignin. The cellulose content in softwood biomass is 18–42%; hemicellulose is 15–27%; lignin content is 30–60%. Regarding hardwood biomass, its cellulose content is higher—22–45%; hemicellulose is 20–38%, and the lignin content is 20–55% [
12,
13]. Cellulose and hemicellulose are polysaccharides consisting of different fermentable carbohydrate monomers such as glucose, galactose, xylose, arabinose, etc. In contrast, lignin has a complex molecular structure of cross-linked polymer chains from phenolic monomers that can be used for the production of chemicals and other purposes [
14,
15,
16].
The pretreatment of biomass is a significant step in bioethanol production. To this end, the combination of physical milling and biodegradation of the recalcitrant lignocellulosic matrix is considered an environmentally friendly technology. Also, this pretreatment technology has beneficial effects in reducing biomass crystallinity and rigidity [
15,
16,
17]. For example,
Trichoderma reesei (
T. reesei) is a filamentous mesophilic soft rot fungus. It is the most widely used commercial-scale fungus to produce cellulase and hemicellulase enzymes.
T. reesei hydrolyzes polysaccharides via its extracellular secreted enzymes. Moreover,
T. reesei has demonstrated high production levels of these biomass-degrading enzymes and showed good adaptation to fermenter cultivations [
16,
18,
19]. Previous studies showed that
T. reesei can depolymerize lignin, decrease cellulose crystalline structure, and hydrolyze hemicellulose [
16,
20]. Thus,
T. reesei enables the release of fermentable monosaccharides which are normally utilized by other micro-organisms [
16]. The yeasts which are most widely used in the industry to ferment monosaccharides into ethanol are
Saccharomyces cerevisiae (
S. cerevisiae) and
Pichia stipitis (
P. stipitis). These yeasts are highly productive and tolerant to bioethanol released into the medium via the fast and simple glucose and xylose fermentation pathways [
21,
22].
Currently, the main challenges of bioethanol production are the poor recovery of biocatalysts and insufficient operational stability. These challenges may be met by immobilizing the yeast cells. Recently, many studies have been published comparing suspended planktonic cultures and immobilized systems [
23,
24,
25]. Many of these studies report significant benefits of immobilized cell application due to improved immobilized molecule recovery, better activity and easy separation of immobilized cells or biocatalysts, increased stability, and better selectivity of biocatalysts, effective separation of products, and purification steps. Moreover, employing cell immobilization methodologies reduces total costs and increases the overall efficiency and volumetric productivity of bioethanol [
25]. However, there are disadvantages as well. Some of the specified difficulties are the lower metabolic activity experienced by immobilized cultures. Metabolic activity decrease results from lower nutrient and oxygen availability due to lower diffusion rates through the encapsulation matrix, high density of cells, and lack of carrying material recycling ability. In addition, the repeated fermentation processes with immobilized cells indicated that carrying matrices degrade after a low number of fermentation cycles [
26,
27].
Various encapsulation and immobilization techniques have been used in research and industrial processes that were based on matrices with various physical and chemical properties [
28]. These immobilization carriers were found to increase system stability over a broad spectrum of environmental conditions, enable easy separation recycling of the encapsulated cells for continuous operation, produce a pure product, and reduce total costs [
25]. On the other hand, several concerns are associated with cell immobilization such as the high cost of carrier materials, possible cell leaks, and limited diffusion of substrates and products. Furthermore, the secreted cellular metabolic products may affect the structural integrity of the matrices [
29,
30]. Hence, there is a need to develop an innovative approach that may offer enhanced stability, activity, selectivity, higher resistance against inhibition and environmental pollutants, and prolonged viability [
25,
31].
Recently, a novel biotechnological method was suggested, termed “Small Bioreactor Platform” technology (SBP). This method was developed to improve exogenous micro-culture adaptation, metabolic activity, nutrients and oxygen diffusion rates, microbial biomass protection, and maintenance over time [
32]. SBP is based on the encapsulation of microbial cells in macro-capsules made from structural microfiltration membranes. These macro-capsules isolate the cells within a sustainable confined environment, thus shielding the cells from the external natural microbiota and problematic conditions that may cause undesired effects such as withdrawal activity. SBP technology enables the preservation of a high concentration of cultures within a sustainable internal environment. The SBP technology does not require cell immobilization into carriers or adsorption to gelatinous matrices, and the introduced cultures are in a free suspended state inside the capsules. In addition, this technique provides higher permeability rates of dissolved nutrients and oxygen compared to polymeric beads [
32,
33,
34].
According to studies conducted on the architecture of the SBP capsules, the structure of the membrane was determined [
34]. It has been shown that the SBP’s unique membrane provides a an interesting biodegradation scenario: the differently sized molecules take a fast path by funnel-like pores beside slower diffusion through the nano-mesh structure due to the concentration gradient [
34]. Thereby, constant flow and variability of substrate components can be achieved. We hypothesized that the penetration mechanism of substrates and metabolic products will induce simultaneous saccharification and fermentation processes. Additionally, the same microorganisms can be reused for longer periods.
In the present work, our purpose was to perform a continuous bioethanol production process from commercial xylan (from corn core) and cellobiose by encapsulating T. reesei, S. cerevisiae, and P. stipitis in SBP capsules. To this end, we investigated the effects of encapsulating these biocatalyst cultures on their viability post encapsulation, biological activity, and bioethanol production. We have also evaluated the longevity of the system for a period of up to three months. Two different configurations were tested. The first one consisted of columns packed with T. reesei fungal cells and specific yeast cells together. In the second configuration, the fungal cells were separated from the yeast cells into two columns in series.
2. Materials and Methods
2.1. Substrate Preparation
Xylan from corn core and cellobiose, purchased from Acros Organics, Belgium, were used as substrates. Sterile sugar stock solutions were prepared at a concentration of 5 g L−1. Then, 10 mL of 3 mg mL−1 chloramphenicol (Sigma Aldrich, St. Louis, MO, USA) in 50% ethanol (Merck, Darmstadt, Germany) was added to 1 L of sugar stock solutions to prevent bacterial contaminations.
2.2. Strains and Maintenance
The fungus T. reesei QM 9414 was obtained as a dry spore powder from Leibniz Institute DSMZ, Braunschweig, Germany. The dry spore powder was suspended in a preprepared sterile growth medium. The suspension was maintained aseptically on potato–dextrose agar (PDA) (Difco, Franklin Lakes, NJ, USA) slants. The PDA slants were incubated at 30 °C for 7 days until good sporulation was reached and then stored at 4 °C.
S. cerevisiae 70,468 was also acquired from Leibniz-Institute DSMZ, Germany. P. stipitis NRRL Y-7124 was purchased from The Global Bioresource Center ATCC, Gaithersburg, MD, USA. Stock yeast precultures for continuous experiments were maintained on YMA (Yeast Malt Agar) (Difco, Franklin Lakes, NJ, USA) plates containing: 3 g L−1 of yeast extract, 3 g L−1 of malt extract, 5 g L−1 of peptone, 10 g L−1 of dextrose, and 20 g L−1 of agar at 30 °C for 48 h. Then YMB (Yeast Malt Broth) slants with colonies were stored at 4 °C.
2.3. Inoculums Preparation
T. reesei conidia were harvested from 14 to 30 day old stock agar slants, by adding 6 mL of sterile growth medium. Resuspended conidia were used to initiate growth in 750-mL Erlenmeyer flasks with 150 mL of nutrient medium composed of: 6.4 g L−1 K2HPO4 (Merck, Darmstadt, Germany); 5.0 g L−1 (NH4)2SO4 (Merck, Darmstadt, Germany); 0.34 g L−1 MgSO4∙H2O (Sigma Aldrich, St. Louis, MO, USA); 0.6 g L−1 CaCl2 (Fluka, Germany); 5.0 mg L−1 FeSO4∙7H2O (Sigma Aldrich, St. Louis, MO, USA); 1.6 mg L−1 MnSO4∙H2O (Fluka, Germany); 2.24 mg L−1 ZnSO4∙7H2O (Sigma Aldrich, St. Louis, MO, USA); 2.0 mg L−1 CoCl2∙6H2O (Sigma Aldrich, USA); 0.2 mL L−1 Tween-80 (Merck, Germany); 0.75 g L−1 peptone (Merck, Germany); 0.3 g L−1 yeast extract (Neogen, Lansing, MI, USA); and 30 g L−1 lactose monohydrate (Acros Organics, Geel, Belgium). Lactose has been used as the carbon source and inducer of industrial enzyme production in T. reesei. Inoculums were incubated on a rotary shaker with an agitation speed of 200 rpm at 30 °C for 5 days. At the end of the cultivation period, samples were harvested and centrifuged for 5 min at 10,000 rpm and 4 °C. The purified culture supernatants containing glycosidases and residual suspended fungus spores were immediately immobilized and used in the continuous fermenter.
S. cerevisiae and P. stipitis yeast colonies were transferred separately from the stock culture plates into glass tubes containing 5 mL of YMB (Difco, Franklin Lakes, NJ, USA) sterilized medium. Prepared inoculums were grown at 30 °C for 24 h with an agitation speed of 200 rpm in an orbital shaker before immobilization and subsequent use in a continuous system.
2.4. Shake-Flask Experiments
The enzymatic complex activity produced by T. reesei was examined in 150 mL wide mouth Erlenmeyer flasks. Separately, 5 mL aliquots of fungus inoculum were used to initiate growth on secondary media based on 10 g L−1 xylan or 10 g L−1 cellulose as a single carbon source. Each series contained 45 mL of specific growth medium which was grown on the lactose-based substrate with 5 mL of pre-harvested culture supernatant. To each Erlenmeyer flask was added 0.5 mL of 3 mg mL−1 chloramphenicol at 50% ethanol solution to prevent contamination (the final antibiotic concentration was 30 μg mL−1). The following cultivation parameters for xylanase activity were selected: temperature of 37 °C agitated at 200 rpm for 14 days. As for cellulase activity, the cultivation flasks were incubated at 30 °C agitated at 200 rpm for 14 days. Samples were withdrawn daily and analyzed for their polysaccharides and monosaccharides composition via HPLC. Dionex Ultimate 3000 (Thermo Scientific, Karlsruhe, Germany) system was used with CAD—Charge Aerosol Detector—Corona Ultra RS (Thermo Scientific, Karlsruhe, Germany). The Phenomenex Rezex RPM-Monosaccharide Pb+2 (8%) with a 300 × 7.8 mm column was selected for analysis. Water was deionized and further purified for HPLC with the Milli-Q system, Millipore, Burlington, MA, USA. The mobile phase flow rate was set to 0.6 mL min−1. The column temperature was kept at 75 °C, while the sample was kept at 25 °C. The injection volume of the samples was 2 µL.
2.5. The Immobilization of Micro-Organisms Using SBP Technology
The SBP cylindric capsules (2.5 cm in length; 0.8 cm in diameter) were purchased from BioCastle Water Technologies (Afula, Israel). The structure, composition, and validation of the SBP capsules are detailed by the producer [
32].
The encapsulation procedure was conducted as described by BioCastle Water Technologies [
32]. Briefly, the SBP macro-capsules were supplied in a dry inactivated state. First, the SBP capsules were activated by encasing the suspended fungus spores within. The activation process occurs in several steps (according to the producer). For that purpose,
T. reesei conidia were harvested as described above (
Section 2.3). The supernatant containing the spores was injected into the inner capsule space, using a syringe and needle of MEDI-PLUS (Gujarat, India) and KDL (Shanghai, China), respectively. Thereafter, the capsules with inserted suspension were transferred into Erlenmeyer flasks containing 60 mL of saline supplemented with 0.6 mL of 3 mg mL
−1 chloramphenicol solution in 50% ethanol (final antibiotic concentration of 30 μg mL
−1). Initial incubation continued for 24 h at 30 °C and 200 rpm. Subsequently, these capsules were transferred into another Erlenmeyer flask containing 60 mL of saline supplemented with a 2%
T. reesei-specific lactose-based growth. Additional incubation continued for 48 h at 30 °C and 200 rpm. After 72 h, the activated capsules were transferred into Erlenmeyer flasks containing 60 mL of 3% lactose-based fungal growth medium and incubated for 5 days at 30 °C and 200 rpm.
A similar activation and initial growth procedures were applied during the preparation of capsules containing the yeast cells. The two yeast strains were cultured separately: 1.5 mL were taken from yeast inoculums prepared previously and inserted into the SBP capsules. Initial cultivation was performed for 24 h at 30 °C and 200 rpm in 60 mL saline supplemented with 0.6 mL of 3 mg mL−1 chloramphenicol solution in 50% ethanol (final antibiotic concentration was 30 μg mL−1). As a continuation of the activation process, the capsules were transferred into new Erlenmeyer flasks containing 60 mL of saline supplemented with 2% YMB and 30 μg mL−1 chloramphenicol in 50% ethanol. The Erlenmeyer flasks were placed in an incubator for 48 h at 30 °C and 200 rpm. After 72 h, the SBP capsules with yeast cells were transferred into separate Erlenmeyer flasks containing 60 mL of YMB for an incubation period of 2 days at 30 °C and 200 rpm.
2.6. Continuous Simultaneous Reactor Design
A two-step bioreactor was designed to perform continuous saccharification and fermentation processes for bioethanol production. Two configurations were constructed, each one based on a different substrate solution and micro-organism composition performing the saccharification and fermentation steps. In each configuration, the scheme included a horizontally oriented column filled with SBP capsules with encapsulated cells. Substrate solutions were through the columns using a peristaltic pump (ISM1089, Ecoline Ismatec, Wertheim, Germany). At the outlet from the column, the fractions were collected and analyzed for product and substrate concentration.
The first bioreactor system was designed to examine the ability of the encapsulated cells to perform the simultaneous saccharification and fermentation processes of commercial xylan and cellobiose sugars. Our proposed system setup is presented and depicted in
Figure 1.
The feed flow rate from each stock solution (10 g L−1 xylan/cellobiose) was controlled by a peristaltic pump, adjusted to 0.6 mL min−1, and supplied to the packed columns (Econo-Column Chromatography; 20 cm length; 1.5 cm ID) via pump tubing (Ismatec TYGON 3-stop; 1.02 mm ID, Ismatec, Wertheim, Germany). The feed flow rate was restricted by the pump’s capabilities and in order to avoid pipes’ clogging from substrate sedimentation. Also, this minimal flow enabled the longest residence times in the columns. The residence time of the substrate in the column, calculated from the free volume of the reactor—40 min. The xylan-based medium was supplied to the reactor with encapsulated T. reesei cells, where the capsules were placed near the inlet to the column to allow saccharification. The xylan-to-xylose conversion was achieved by enzymatic digestibility of xylan portions. SBP capsules with P. stipitis cells were located sequentially to provide fermentation of xylose units. Commercial cellobiose was used as the carbon source for T. reesei in the second column, which was packed with S. cerevisiae encapsulated cells. Thus, cellobiose-to-glucose conversion occurred, and glucose fermentation was carried out sequentially. Fractions from each column were collected daily. Samples were withdrawn daily and analyzed for their polysaccharides and monosaccharides composition via HPLC.
The concentration of bioethanol in the collected fractions was analyzed by Headspace Gas Chromatography (HS-GC). The following system was used: Master GC device with Flame Ionization Detector—FID, combined Master PAL Combi-xt autosampler (PAL system, Zwingen, Switzerland), Rtx column (30 m × 0.53 mm; 1.5 µm) (Restek, Bellefonte, PA, USA) and calculated using the internal standard method. Propanol (BioLab Ltd., Jerusalem, Israel) was used as an internal standard. A PG-H2 100 plus type hydrogen generator (LNI Schmidlin SA, Switzerland) was used for FID maintenance.
The second bioreactor model was assembled in the same manner as described above, except for these parameters: 5 g L
−1 xylan-based substrate was transferred to the first column packed with
T. reesei cells only. Afterward, the substrate was transferred into the second column packed with
P. stipitis cells only. The fractions were withdrawn at the outlet from each column daily. Then, collected fractions were analyzed for their polysaccharides and monosaccharides composition via HPLC. Additionally, collected samples were tested for the presence of ethanol by GC, with their concentration calculated over time. The xylose fermentation ability of encapsulated
T. reesei cells and the contribution of yeast cells to produce bioethanol were tested this time. The constructed system is depicted in
Figure 2.
The efficiency (
η) of each column was calculated using the following equation:
where m(final EtOH) is the average bioethanol mass produced in each column. Calculation of this value was based on the daily substrate flow, the molecular weights of glucose or xylose, and glucose or xylose found within the polysaccharide chain. m(theoretical EtOH) is the theoretical bioethanol mass produced from a complete conversion of glucose or xylose (i.e., 0.51 g bioethanol and 1 g glucose or xylose, respectively). The stoichiometric equations of glucose and xylose conversion to ethanol are presented by Equations (2) and (3) respectively:
2.7. Statistical Analysis
The results were obtained from at least three independent experiments carried out in duplicates and analyzed by single-factor ANOVA analyses. The Analysis ToolPak in Excel was used for the statistical analysis. Quantitative results are presented as the means ± standard error. The difference between results was considered significant when the p-value was less than 0.05.
3. Results and Discussion
3.1. Assessing Fungal Activity in a Batch Bioreactor
At first, batch experiments were performed to test the ability of the fungus
T. reesei to degrade commercial polysaccharides (xylan and cellobiose) into fermentable carbohydrate monomers. The fungus was grown separately on two similarly prepared media—containing either xylan or cellulose as the carbon source. Lactose was used as a carbon source and inducer for cellulase and xylanase secretion in the pre-cultivation step. Biomass formation of cultured
T. reesei on PDA (Potatozx–Dextrose Agar) plates and in a shaken batch bioreactor are shown in
Figure 3.
One-week-old green shade conidia were formed on PDA plates with yellow pigment secreted into the agar (
Figure 3a,d). The freely dispersed fungal mycelia were homogeneous, thin, and consisted of large and loose yellowish colonies mainly composed of entangled branched into broth substrate (
Figure 3b,c,e,f). Next, we measured the release of cellulase and hemicellulase enzymes into the growth media over 15 days by assessing the degradation of the polysaccharides (xylan and xylose) via HPLC analysis. The results are presented in
Figure 4.
The results of the batch initial experiments suggest high rates of glycosidases activity of
T. reesei. It was impossible to monitor the cellulose concentration variability in the medium, since it is insoluble in water. However, glucose formation from cellulose decomposition was observed. Thus, the results of glucose were presented graphically. The glucose appeared in the solution only after 24 and 48 h of incubation (
Figure 4a). Glucose is consumed by the fungal cells immediately and efficiently as an accessible carbon source. Its maximum concentration was obtained after 24 h of incubation reaching 0.65 g L
−1 (
Figure 4a). After 48 h its concentration dropped significantly until it was undetectable. As can be seen in
Figure 4b,
T. reesei consumed efficiently both types of sugars—xylan and xylose. The hydrolysis of xylan was observed first suggesting that the enzyme xylanase was released first into the medium. Xylose consumption was detected after fungal adaptation to the substrate composition. The appearance of the additional carbon source stimulated additional appropriate enzymatic secretion. The resulting low concentrations of monosaccharides—glucose and xylose—can be explained by rapid consumption by
T. reesei.
3.2. One-Step Continuous Bioreactor Operation Fungal Activity in a Batch Bioreactor
Once the feasibility of the proposed system in a batch operation was established, we moved to the design of the continuous set up to describe and predict the decomposition of lignocellulosic polymers and for the complete conversion of glucose or xylose monomers by encapsulated microorganisms. Three milestones were set to evaluate the behavior of cells in the submitted protective shell such as (1) diagnosis of the effect of capsules on proliferation and metabolic activity of the cells; (2) the possibility of a successful combination of both processes in the same reactor—enzymatic saccharification and monosaccharides’ conversion to bioethanol; (3) testing the ability of T. reesei to ferment xylose and to produce bioethanol. Briefly, micro-organism cells were kept inside the semi-permeable membrane under favorable conditions for a few days before being transferred to the reactor columns. During the pre-activation process, the capsules were kept in saline solutions supplemented with 0.6 mL of 3 mg mL−1 chloramphenicol solution in 50% ethanol. Thus, the activated capsules with fungi and yeast cells were inserted into the columns with an initial amount of ethanol.
Two different continuous reaction models were designed and evaluated in this study (
Figure 1 and
Figure 2). We studied the efficacy of simultaneous cultivation of yeasts and fungal cells. Encapsulated
T. reesei cells were located at the entrance to each column to ensure that the degradation of the polysaccharides occurs first. Sequentially, SBP capsules—with
S. cerevisiae cells—were used in the upper column to utilize glucose produced from cellobiose saccharification. The second xylan-based column contained encapsulated
P. stipitis cells for xylose-based hydrolysates fermentation.
First, the adaptability of the micro-organisms to the novel SBP encapsulation technique was estimated. For that reason, simultaneous enzymatic hydrolysis of polysaccharides and subsequent fermentation of monosaccharides were performed in the one-step reactor, the major components of which are depicted in
Figure 1. Media solutions were supplied to the bioreactors using a peristaltic pump. The samples for HPLC (to check the sugar content in the substrate) and GC (to monitor the bioethanol concentration) analyses were collected from each column for over two months. Both cellobiose and xylan concentrations decreased insignificantly over time. Monosaccharides were not detected during the experiment. The obtained results are presented graphically in
Figure 5.
As can be seen in
Figure 5, only partial utilization of both—the disaccharide cellobiose and the polysaccharide xylan—occurred. Relatively short residence times of the substrate molecules resulted in minor utilization. In addition, the amount of SBP capsules with
T. reesei cells packed in the column could also affect the hydrolysis of cellobiose and xylan.
Bioethanol concentrations were measured accurately with a GC-based method as described above. The appearance of bioethanol in collected fractions from day one can be explained by the pre-activation treatment of SBP capsules (
Section 2.5). Furthermore, the diffusion of substrate molecules (i.e., cellobiose and xylan) across the capsule membrane enabled a biodegradation rate. The micro-environment within the SBP capsules enabled fermentation. Bioethanol production continued throughout the experiments (>60 days) as shown in
Figure 6. Maximum fermentation time that has been reported by various studies—in both batch and continuous systems—usually did not exceed a few days (36–60 h) [
22,
35,
36].
Figure 6 shows the bioethanol concentration profile during the continuous process experiments. The cellobiose- and xylan-based systems showed an upward trend in bioethanol concentration until the 16th–17th day of the process. Subsequently, both systems reached a steady-state, and the bioethanol concentration remained constant for an additional 42 days. More specifically, for an inlet cellobiose concentration of 10 g L
−1 at an inflow rate of 0.6 mL min
−1, a maximum amount of 4.1 g L
−1 of bioethanol was obtained for several incubation periods—day 16, day 38, days 48–49, and day 59 (
Figure 6a). The average bioethanol concentration at a steady-state was 3.6 ± 0.5 g L
−1. Yadav et al. (2011) reported that a culture of
S. cerevisiae in a batch mode utilized 30 g L
−1 of sugars and produced ethanol in a maximal concentration of 7.5 g L
−1 in 36 h [
36]. For the xylose utilization by
P. stipitis, a similar experiment was carried out using a 10 g L
−1 xylan-based substrate medium at an inflow rate of 0.6 mL min
−1. The results show that a high 3.7 g L
−1 concentration of bioethanol was achieved at 36 and 53 incubation days (
Figure 6b). Data points represent the average bioethanol concentration of 3.6 ± 1.4 g L
−1. In the work of Yong et al. (2012) the maximal ethanol concentration obtained by
P. stipitis grown on a proliferation medium using a rotary shaker incubator for 36 h was 10.3 g L
−1 [
37]. It was also reported that adapted co-culture of immobilized
S. cerevisiae and
P. stipitis generated 4.4 g L
−1 and 5.0 g L
−1, respectively. According to Lee et al. (2013), the maximal operation time of immobilized cells was 21 days only [
22].
According to the efficiency calculations, the cellobiose-to-ethanol conversion was η = 67.5% while the efficiency for xylan-to-ethanol conversion was η = 62.5%.
The results obtained from the first continued bioreactor indicate that the SBP technology can increase the stability of the biological process in suboptimal cultivation conditions. Also, the simultaneous activity of the fungal and yeast cells was allowed in a common reactor. In this case, the efficiency for cellobiose and xylan conversion to bioethanol was calculated to be η = 60–70%. The duration of this process, which was over 60 days without supplementing it with fresh cells, was very impressive and serve as a great starting point and proof of the concept.
3.3. Step-by-Step Continuous Bioreactor
Subsequent studies have been performed on a similarly designed continuous bioreactor with two columns positioned one after the other (fungus column first) with a xylan-based growth medium. This substrate was chosen for two reasons: (1) xylans are one of the types of hemicellulose and are found in lignocellulosic residues. In contrast, cellobiose is a building unit of cellulose. The aim was to show the decomposition ability of biomass components by fungal cells. (2) We wanted to test the survivability of P. stipitis yeast in SBP capsules, and their ability to ferment xylose in defined environmental conditions.
Encapsulated T. reesei cells were placed in the first column to hydrolyze the polymeric matrix of xylan. The second column was packed with P. stipitis yeast cells to convert the xylose units—obtained from fungal activity—to bioethanol. The xylan-based substrate (5 g L−1) feed was set by a peristaltic pump. The samples for HPLC and GC analyses were collected from each column for over 12 weeks.
Substrate composition from both reactors was analyzed by HPLC. The decomposition of xylan was detected only. Xylose was not detected due to the fact of its immediate utilization by both the fungal cells and yeast cells. The obtained results are presented graphically in
Figure 7.
As can be seen in
Figure 7, for the first 20 days of the process, the xylan concentration remained the same (5 mg mL
−1 for the column packed with encapsulated
T. reesei and 4.5 mg mL
−1 for the column packed with encapsulated
P. stipitis) with negligible xylan consumption. Subsequently, a downward trend was observed in the concentration of xylan for both setups. From the data in both graphs, it can be concluded that the significant xylan saccharification was detected in the first column with encapsulated
T. reesei cells (
Figure 7a). More specifically, for a xylan inlet concentration of 5 g L
−1 at an inflow rate of 0.6 mL min
−1, a decrease in the polysaccharide concentration to an average value of 3.8 g L
−1 was obtained after 20 days (
Figure 7a). Data points represent the average xylan concentration of 3.8 ± 1.1 g L
−1. When comparing xylan concentration in the second column (
Figure 7b), a similar average value of 3.7 ± 1.0 g L
−1 was obtained. Therefore, based on the results obtained, partial substrate utilization has occurred in both columns, with additional negligible consumption occurring in the column packed with
P. stipitis. It is assumed that the inlet substrate concentration was too high for the number of encapsulated cells used, resulting in low yields.
The production of bioethanol from this setup was examined by GC analysis.
Figure 8 shows bioethanol concentration in both columns over incubation time.
As seen in
Figure 8, bioethanol production lasted for about 2.5 months, then reduced to zero within a few days. When applying a xylan inlet concentration of 5 g L
−1 at an inflow rate of 0.6 mL min
−1, data points represent the average bioethanol concentration of 0.6 ± 0.4 g L
−1 from the column packed with encapsulated
T. reesei. Data points from the column packed with encapsulated
P. stipitis represent the average bioethanol concentration of 0.614 ± 0.3 g L
−1. The appearance of bioethanol in collected fractions from day one can be explained by the pre-activation treatment of SBP capsules (
Section 2.5). Briefly, during the pre-activation process, the capsules were kept in saline solutions supplemented with 0.6 mL of 3 mg mL
−1 chloramphenicol solution in 50% ethanol. Thus, the activated capsules with fungi and yeast cells were inserted into the columns with an initial amount of ethanol.
The results obtained, which are presented in
Figure 8, suggest that xylose fermentation by encapsulated fungi cells affected bioethanol production by yeasts. Immediate utilization of xylose by
T. reesei created a low-carbon environment in the growth medium for
P. stipitis. Thus, bioethanol addition from the second column with
P. stipitis was negligible. Another possible explanation for the achieved low bioethanol concentration in the second column could be that when xylose is unavailable, other substrates such as bioethanol can be used by yeast cells. At the same time, the results indicate the appearance of bioethanol in the column with the fungal cells. As such, this proves the ability of
T. reesei to ferment xylose to produce the desired biofuel.
The efficiency for xylan-to-ethanol conversion in the step-by-step bioreactor was η = 13.3%. The efficiency calculation was performed for the ethanol concentration obtained from the second column packed with P. stipitis. A significant amount of ethanol was obtained in the first column, while the effect of the additional ethanol from the second column was negligible. It was assumed that the separation of the microorganisms would lead to efficient utilization of xylan, higher decomposition percentages, and larger amounts of the fermentation product. However, the results indicate a decrease in system efficiency. In the first setup, P. stipitis cells were able to utilize more significant portions of xylose, thus producing a higher amount of bioethanol. T. reesei cells are capable of fermenting xylose, but the amounts of bioethanol obtained from the fermentation are significantly lower.
The second reactor was designed to test the ability of T. reesei cells to ferment monosaccharides (xylose) and to examine the maximum viability duration of the micro-organisms inserted into the capsules. The results obtained from HPLC and GC analyses showed xylan-to-ethanol conversion into the column packed with encapsulated T. reesei cells. Additionally, the formation of a carbon-poor environment was found in the serial column packed with encapsulated P. stipitis. Thus, bioethanol addition from the second column with P. stipitis was negligible. It was concluded that separating the micro-organisms into two different columns was less effective than simultaneous use. The viability of this setup was even longer this time and prolonged 85 consecutive days without adding fresh cells.
4. Conclusions
The potential of SBP encapsulation technology for lignocellulosic polysaccharide biodegradation and bioethanol production was evaluated. This new approach enabled us to implement the exogenous specific microculture insertion—as whole organisms, without causing physiological change—within a host mini-bioreactor in our designed continuous system setup. The fungus and yeast cells inside the SBP capsules were in a suspended state within the specific growth medium and not fixed either by adhesion or entrapment into any solid surface or inside the gelatin matrix. The physical encapsulation of a confined environment and unrestricted aquatic medium with essential nutrients provided rapid acclimation and micro-culture proliferation and prosperity for at least several weeks.
Another significant benefit is the availability of simultaneous saccharification and fermentation in the same reactor over a very long time—approximately 2.5–3 months—with no need to renew the cells or the carried matrices. The purpose of this study was to establish a continuous bioethanol production process by improving the overall activity of microorganisms and by allowing simultaneous saccharification and fermentation. The goal was achieved, with a system efficiency of 60–70%, evidence of long-term prosperity and uninterrupted metabolic activity. Along with high efficiency percentages, low yields were obtained due to the use of low substrate concentrations. Additional experiments should be performed with higher concentrations of commercial substrates. The results of this study provide proof of the concept that SBP technology can be an immensely powerful game changer and lead to a breakthrough in the field of alternative energies.
It was assumed that the minimal flow rate will result in long residence time. Previously, the optimal residence time needed to obtain maximum productivity in continuous fermentation was reported to be 7.47 h [
35]. The productivity of a single continuous flow system is a function of the residence time of the substrate in the reactor. Long residence time allows for prolonged exposure of microorganisms to the supplied substrate, thus yielding increased conversion. Thus, a systemic tuning of the residence time can be used to control the yield of the process by determining the reaction rate and the conversion of the substrate. Therefore, additional feed flow rates should be evaluated. By applying the longer residence time, the low production of bioethanol may significantly improve.
Further studies will check the decomposition of solid lignocellulosic biomass instead the commercial liquid substrates. The feedstock pretreatment by physical methods will be carried out to achieve biomass suspension. The main purposes of using SBP capsules were to isolate the microorganisms from the substrate and prevent them from washing out of the system, without the need to immobilize them. Cellulase and hemicellulase are extracellular enzymes, with a diameter and a total enzyme length of a few nanometers. The size pore of the SBP capsule membrane was 0.75 µm, as reported by the manufacturer. Thus, the transmission of enzymes through the membrane enabled their interaction with solid particles of biomass suspension will occur.
In conclusion, it is important to note that at the end of the study, the SBP capsules did not show any structural changes (i.e., membrane crystallization, colloidal fragments inside the capsule, capsule swelling, or membrane degradation). Additionally, the visual test showed no changes to fungal mycelium or yeast colonies grown on PDA or YMA that slants, respectively. The present study pointed out that the innovative SBP technology makes it possible to avoid the need to immobilize the micro-cultures into a biological or chemical matrix. This approach enabled us to introduce the living cells with a specific growth medium to the capsules. On the one hand, there is an opportunity to work with cells in suspended form, while, on the other, the presented system has many advantages of immobilized cells. Also, the enabled combination of two process steps and prolonged use of micro-organisms—without the extending action of viability—results in a lower capital cost and the possibility to achieve complete conversion of fermentable monosaccharides to bioethanol. The biofuels production field aims to design more effective, low-cost, and eco-friendly processes, which will provide an alternative solution for worldwide fossil fuel demand. The perfect solving strategy for defined parameters is based on the maximum use of natural resources in their raw form. Consumption of lignocellulosic biomass alongside the use of microorganisms—without complicated enzyme extraction processes or recombinant strain creation—can lead to sustainable development and optimization of green energy production processes. Further studies should be carried out to further characterize the metabolic behavior of encapsulated microorganisms at additional inflow concentrations, flow rates, and configurations.