1.1. Critical Heat Flux in Pool Boiling
Pool boiling is a common phenomenon, where phase-change takes place in a pool of macroscopically stationary liquid without the presence of external flow, which would affect the heat transfer phenomena. Engineering applications mostly rely upon the nucleate boiling regime of pool boiling, where high values of the heat transfer coefficient are achievable at relatively small temperature differences between the boiling surface and the liquid bulk. The upper heat flux limit of the nucleate boiling regime is called the critical heat flux (CHF), where the entire boiling surface becomes covered with a vapor film acting as a thermal insulator. This severely decreases the heat transfer intensity during the ensuing transition towards film boiling, and is mostly undesirable. Many widespread technologies rely on boiling heat transfer and tend to operate at high heat flux values (e.g., nuclear reactors, steam boilers, microelectronic cooling systems etc.), making accurate prediction of the CHF value paramount to ensure their safe and efficient operation.
1.2. Existing CHF Correlations
As CHF incipience in a practical application could severely damage equipment and cause process failure, it is imperative to avoid it. For that purpose, one must be able to estimate at what heat flux the CHF might occur to be able to safely dimension the equipment and the boiling process. The first CHF correlation was proposed in the 1950s by Kutateladze [
1], who correlated the critical heat flux to be a function of liquid and vapor density (
ρl and
ρv), surface tension
σ, gravitational acceleration
g and latent heat of vaporization Δ
hlv alongside a constant
K:
Kutateladze proposed the empirically obtained value of the constant K (also known as the Kutateladze number) to be 0.16.
Later in the same decade, Zuber [
2] developed a correlation for estimating the CHF value, which is based on the hydrodynamic instability theory, and is arguably the most well-known CHF correlation to date. In derivation of his theory, Zuber assumed that Taylor’s and Helmholtz’s instability were the main factors behind the CHF incipience. Vapor jets on the surface were assumed to be spaced on a square grid in accordance with the Taylor’s wavelength, whereas instability of the vapor jets was assumed to be governed by the Helmholtz’s wavelength, stemming from the vapor velocity becoming too great. Zuber assumed that the jet diameter is one half of the most dangerous Taylor’s wavelength, and theoretically derived the same expression as Kutateladze [Equation (1)], but with a different value of the constant (i.e., the Kutateladze number), which was to be π/24 (approximately 0.131). We will refer to the correlation given in Equation (1) as the Kutateladze–Zuber correlation in the rest of the manuscript.
The CHF correlation was further developed by Lienhard and Dhir [
3], who observed that the CHF value is also dependent upon the heater geometry. Through modification of Zuber’s theory, Lienhard and Dhir obtained a Kutateladze number of 0.149 for an infinite flat plate heater. They also found that CHF appears to increase on small heaters [
4], which was shown to depend on the ratio between the heater’s characteristic dimension and the so-called capillary length
b, calculated as:
Alternative theories on the reason for CHF incipience have also been proposed. For example, dryout of the liquid macrolayer formed below large coalesced bubbles might be blamed for CHF onset [
5,
6].
Several correlations for predicting the CHF value were proposed since the initial publications by Kutateladze and Zuber. The latter correlation only takes into account the hydrodynamic aspects of the CHF incipience, and therefore fails to account for other phenomena, such as the effect of heater size (as mentioned above), heater’s surface properties (such as its wettability, roughness, and wickability), thermal properties of the substrate (e.g., its effusivity) etc. New correlations aim to tackle one or more of these missing aspects to possibly provide a generalized model for CHF description. One such model was proposed by Bar-Cohen’s team [
7,
8,
9]:
The latter correlation includes the Kutateladze–Zuber correlation with an addition of functions that account for the thickness and thermal effusivity [
10] of the heater [
], the dimensionless length of the heater [
, defined in Equation (4) as the ration between the length of the heater and the capillary length], and the liquid bulk’s subcooling [
].
Recent progress in the field of identifying surface properties affecting the CHF value came from Rahman et al. [
11], who found that wickability of the boiling surface represents a key factor in enhancing the CHF value. They propose that a “wicking” Kutateladze number should be defined as:
where
Wi is the dimensionless wicking number. The latter accounts for heat dissipation associated with wicking of liquid across the boiling surface and is calculated through maximum wicked volume flux
(experimentally determined for a given surface) as:
The latter correlation is able to explain both why hydrophilic surfaces offer enhanced critical heat flux, exceeding the Kutateladze–Zuber limit, and that all superhydrophilic surfaces do not offer the same performance due to different wicking volume flux despite the same contact angle value of ~0°.
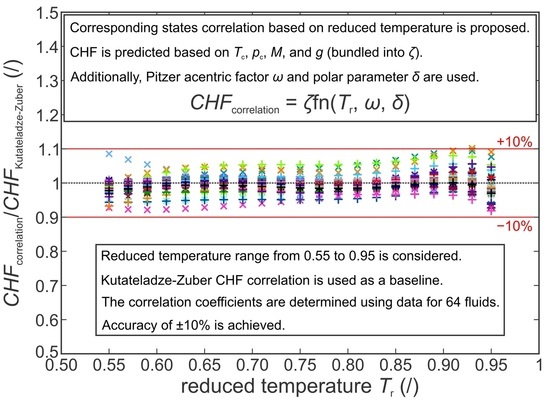
1.3. Corresponding States Correlations
Currently established correlations such as the Kutateladze–Zuber prediction allow for the CHF to be estimated for given experimental conditions by accounting for the fluid properties at the given saturation temperature and pressure. Further improvement of the CHF prediction’s accuracy for functionalized surfaces requires additional parameters to be measures, such as the aforementioned wicking volume flux. However, even the basic Kutateladze–Zuber correlation can be used to estimate the CHF value in dimensioning of common systems involving nucleate pool boiling heat transfer. This, however, requires the user to know the fluid properties, such as the surface tension, liquid and vapor density, and latent heat of vaporization, for any given operating pressure and temperature. While modern databases, such as REFPROP, allow for these values to be easily obtained for many common liquids in any desired state, it can be a hassle to find theirs values for less common or new fluids. Furthermore, they need to be recalculated if the operating point (i.e., saturation pressure or temperature) is changed. Therefore, a generalized description relying on a common behavior of fluids would be beneficial. For this purpose, the corresponding states theorem can be applied to compare the fluids at a given reduced temperature and/or pressure and extrapolate their behavior to other states. Reduced temperature and reduced pressure are defined as the ratio between the given value of the parameter and its critical value (i.e., the critical temperature
Tc or critical pressure
pc):
Several proposition for using corresponding states to correlate the CHF values were made as early as in 1960s [
12,
13]. However, these correlations are based on reduced pressure, whereas reduced temperatures are somewhat more practical for engineering use. Furthermore, the existing correlations based on thermodynamic similarity require data on CHF at a certain reduced pressure, usually at
pr = 0.1 [
14], together with the reduced pressure function or the reduced temperature function [
13], or knowledge of additional group parameters that are hard to determine, such as the Parachor [
12] or the vapor-pressure parameter [
15]. In all cases, the physical properties of the fluid are also required to determine the capillary length given in Equation (2) to adjust the predicted values for finite heaters. Finally, these correlations are based on reduced pressure instead of reduced temperature.