Continuous Production of Lipids with Microchloropsis salina in Open Thin-Layer Cascade Photobioreactors on a Pilot Scale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Climate Simulation
2.2. Microalga Strain and Reaction Medium
2.3. Thin-Layer Cascade Photobioreactor Operation
2.4. Continuous Operation of Thin-Layer Cascades
2.5. Optical Density and Cell Dry Weight
2.6. Total Lipid Analysis
2.7. Urea and Salinity Measurement
2.8. Specific Growth Rate and Biomass Productivity
2.9. Modeling
2.9.1. Kinetics of Microalgae Growth
2.9.2. Kinetics of Lipid Formation
2.9.3. Simulation of Microalgal Growth and Lipid Formation
2.10. Identification of Model Parameters
3. Results and Discussion
3.1. Continuous Lipid Production with Microchloropsis Salina in Thin-Layer Cascade Photobioreactors at Pilot Scale
3.2. Validation of Process Simulations for Biomass and Lipid Production with M. salina
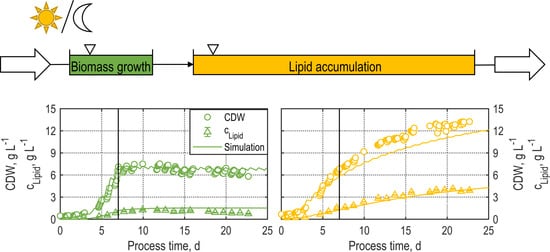
3.2.1. Batch Processes Applying TLC Photobioreactors
3.2.2. Continuous Processes Applying TLC Photobioreactors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohan, S.V.; Hemalatha, M.; Chakraborty, D.; Chatterjee, S.; Ranadheer, P.; Kona, R. Algal biorefinery models with self-sustainable closed loop approach: Trends and prospective for blue-bioeconomy. Bioresour. Technol. 2020, 295, 122128. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Singh, R.; Arora, R.; Sharma, N.K.; Shukla, M.; Kumar, S. Scope of Algae as Third Generation Biofuels. Front. Bioeng. Biotechnol. 2015, 2, 90. [Google Scholar] [CrossRef] [PubMed]
- United Nations General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development, A/RES/70/1. 2015. Available online: https://sustainabledevelopment.un.org/post2015/transformingourworld/publication (accessed on 2 December 2020).
- Moody, J.W.; McGinty, C.M.; Quinn, J.C. Global evaluation of biofuel potential from microalgae. Proc. Natl. Acad. Sci. USA 2014, 111, 8691–8696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Sharmina, M.; Edelenbosch, O.Y.; Wilson, C.; Freeman, R.; Gernaat, D.E.H.J.; Gilbert, P.; Larkin, A.; Littleton, E.W.; Traut, M.; van Vuuren, D.P.; et al. Decarbonising the critical sectors of aviation, shipping, road freight and industry to limit warming to 1.5–2 °C. Clim. Policy 2020. [Google Scholar] [CrossRef]
- Gilbert, P.; Walsh, C.; Traut, M.; Kesieme, U.; Pazouki, K.; Murphy, A. Assessment of full life-cycle air emissions of alternative shipping fuels. J. Clean. Prod. 2018, 172, 855–866. [Google Scholar] [CrossRef]
- Oncel, S.S. Microalgae for a macroenergy world. Renew. Sustain. Energy Rev. 2013, 26, 241–264. [Google Scholar] [CrossRef]
- Slocombe, S.P.; Benemann, J.R. Introduction. In Microalgal Production for Biomass and High-Value Products; Slocombe, S.P., Benemann, J.R., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. xvii–xxx. [Google Scholar]
- Wijffels, R.H.; Barbosa, M.J. An Outlook on Microalgal Biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [Green Version]
- Chisti, Y. Constraints to commercialization of algal fuels. J. Biotechnol. 2013, 167, 201–214. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Chen-Glasser, M.; McMillan, J.D. A perspective on renewable bioenergy from photosynthetic algae as feedstock for biofuels and bioproducts. Algal Res. 2017, 24, 261–264. [Google Scholar] [CrossRef]
- Apel, A.C.; Weuster-Botz, D. Engineering solutions for open microalgae mass cultivation and realistic indoor simulation of outdoor environments. Bioprocess Biosyst. Eng. 2015, 38, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Doucha, J.; Lívanský, K. High Density Outdoor Microalgal Culture. In Algal Biorefineries: Volume 1: Cultivation of Cells and Products; Bajpai, R., Prokop, A., Zappi, M., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 147–173. [Google Scholar]
- De Vree, J.H.; Bosma, R.; Janssen, M.; Barbosa, M.J.; Wijffels, R.H. Comparison of four outdoor pilot-scale photobioreactors. Biotechnol. Biofuels 2015, 8, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grivalský, T.; Ranglová, K.; da Câmara Manoel, J.A.; Lakatos, G.E.; Lhotský, R.; Masojídek, J. Development of thin-layer cascades for microalgae cultivation: Milestones (review). Folia Microbiol. 2019, 64, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Apel, A.C.; Pfaffinger, C.E.; Basedahl, N.; Mittwollen, N.; Göbel, J.; Sauter, J.; Brück, T.; Weuster-Botz, D. Open thin-layer cascade reactors for saline microalgae production evaluated in a physically simulated Mediterranean summer climate. Algal Res. 2017, 25, 381–390. [Google Scholar] [CrossRef]
- Masojídek, J.; Kopecký, J.; Giannelli, L.; Torzillo, G. Productivity correlated to photobiochemical performance of Chlorella mass cultures grown outdoors in thin-layer cascades. J. Ind. Microbiol. Biotechnol. 2011, 38, 307–317. [Google Scholar] [CrossRef]
- Deruyck, B.; Thi Nguyen, K.H.; Decaestecker, E.; Muylaert, K. Modeling the impact of rotifer contamination on microalgal production in open pond, photobioreactor and thin layer cultivation systems. Algal Res. 2019, 38, 101398. [Google Scholar] [CrossRef]
- Montemezzani, V.; Duggan, I.C.; Hogg, I.D.; Craggs, R.J. A review of potential methods for zooplankton control in wastewater treatment High Rate Algal Ponds and algal production raceways. Algal Res. 2015, 11, 211–226. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Mallick, N.; Bagchi, S.K.; Koley, S.; Singh, A.K. Progress and Challenges in Microalgal Biodiesel Production. Front. Microbiol. 2016, 7, 1019. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High Lipid Induction in Microalgae for Biodiesel Production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef] [Green Version]
- Schädler, T.; Cerbon, D.C.; de Oliveira, L.; Garbe, D.; Brück, T.; Weuster-Botz, D. Production of lipids with Microchloropsis salina in open thin-layer cascade photobioreactors. Bioresour. Technol. 2019, 289, 121682. [Google Scholar] [CrossRef] [PubMed]
- Boussiba, S.; Vonshak, A.; Cohen, Z.; Avissar, Y.; Richmond, A. Lipid and Biomass Production by the Halotolerant Microalga Nannochloropsis salina. Biomass 1987, 12, 37–47. [Google Scholar] [CrossRef]
- Severin, T.S.; Apel, A.C.; Brück, T.; Weuster-Botz, D. Investigation of vertical mixing in thin-layer cascade reactors using computational fluid dynamics. Chem. Eng. Res. Des. 2018, 132, 436–444. [Google Scholar] [CrossRef]
- Severin, T.S.; Brück, T.; Weuster-Botz, D. Validated numerical fluid simulation of a thin-layer cascade photobioreactor in OpenFOAM. Eng. Life Sci. 2018, 19, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masojídek, J.; Sergejevová, M.; Malapascua, J.R.; Kopecký, J. Thin-Layer Systems for Mass Cultivation of Microalgae: Flat Panels and Sloping Cascades. In Algal Biorefineries: Volume 2: Products and Refinery Design; Prokop, A., Bajpai, R.K., Zappi, M.E., Eds.; Springer International Publishing: Cham, Switerland, 2015; pp. 237–261. [Google Scholar]
- Byreddy, A.R.; Gupta, A.; Barrow, C.J.; Puri, M. A quick colorimetric method for total lipid quantification in microalgae. J. Microbiol. Methods 2016, 125, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Suh, W.I.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J.-W. Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour. Technol. 2014, 155, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A. Biological Principles of Mass Cultivation of Photoautotrophic Microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; Wiley-Blackwell: Chichester, UK, 2013; pp. 171–204. [Google Scholar]
- Wilhelm, C.; Jakob, T. Balancing the conversion efficiency from photon to biomass. In Microalgal Biotechnology: Potential and Production; Posten, C., Walter, C., Eds.; De Gruyter: Berlin, Germany, 2012; pp. 39–53. [Google Scholar]
- Pfaffinger, C.E.; Schöne, D.; Trunz, S.; Löwe, H.; Weuster-Botz, D. Model-based optimization of microalgae areal productivity in flat-plate gas-lift photobioreactors. Algal Res. 2016, 20, 153–163. [Google Scholar] [CrossRef]
- Pfaffinger, C.E.; Severin, T.S.; Apel, A.C.; Göbel, J.; Sauter, J.; Weuster-Botz, D. Light-dependent growth kinetics enable scale-up of well-mixed phototrophic bioprocesses in different types of photobioreactors. J. Biotechnol. 2019, 297, 41–48. [Google Scholar] [CrossRef]
- Koller, A.P.; Löwe, H.; Schmid, V.; Mundt, S.; Weuster-Botz, D. Model-supported phototrophic growth studies with Scenedesmus obtusiusculus in a flat-plate photobioreactor. Biotechnol. Bioeng. 2017, 114, 308–320. [Google Scholar] [CrossRef]
- Molina-Grima, E.; Camacho, F.G.; Pérez, J.A.S.; Fernández, F.G.A.; Sevilla, J.M.F. Evaluation of photosynthetic efficiency in microalgal cultures using averaged irradiance. Enzyme Microb. Technol. 1997, 21, 375–381. [Google Scholar] [CrossRef]
- Edmundson, S.J.; Huesemann, M.H. The dark side of algae cultivation: Characterizing night biomass loss in three photosynthetic algae, Chlorella sorokiniana, Nannochloropsis salina and Picochlorum sp. Algal Res. 2015, 12, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Janssen, J.H.; Kastenhofer, J.; de Hoop, J.A.; Lamers, P.P.; Wijffels, R.H.; Barbosa, M.J. Effect of nitrogen addition on lipid productivity of nitrogen starved Nannochloropsis gaditana. Algal Res. 2018, 33, 125–132. [Google Scholar] [CrossRef]
- Michels, M.H.A.; Slegers, P.M.; Vermuë, M.H.; Wijffels, R.H. Effect of biomass concentration on the productivity of Tetraselmis suecica in a pilot-scale tubular photobioreactor using natural sunlight. Algal Res. 2014, 4, 12–18. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Inorganic Algal Nutrition. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; Wiley-Blackwell: Chichester, UK, 2013; pp. 123–133. [Google Scholar]
- Keller, M.D.; Kiene, R.P.; Matrai, P.A.; Bellows, W.K. Production of glycine betaine and dimethylsulfoniopropionate in marine phytoplankton. II. N-limited chemostat cultures. Mar. Biol. 1999, 135, 249–257. [Google Scholar] [CrossRef]
- Severin, T.S.; Plamauer, S.; Apel, A.C.; Brück, T.; Weuster-Botz, D. Rapid salinity measurements for fluid flow characterisation using minimal invasive sensors. Chem. Eng. Sci. 2017, 166, 161–167. [Google Scholar] [CrossRef]
- Wang, C.; Lan, C.Q. Effects of shear stress on microalgae—A review. Biotechnol. Adv. 2018, 36, 986–1002. [Google Scholar] [CrossRef]
- Scarsella, M.; Torzillo, G.; Cicci, A.; Belotti, G.; De Filippis, P.; Bravi, M. Mechanical stress tolerance of two microalgae. Process Biochem. 2012, 47, 1603–1611. [Google Scholar] [CrossRef]
- Torzillo, G.; Sacchi, A.; Materassi, R.; Richmond, A. Effect of temperature on yield and night biomass loss in Spirulina platensis grown outdoors in tubular photobioreactors. J. Appl. Phycol. 1991, 3, 103–109. [Google Scholar] [CrossRef]
- Schädler, T.; Neumann-Cip, A.-C.; Wieland, K.; Glöckler, D.; Haisch, C.; Brück, T.; Weuster-Botz, D. High-Density Microalgae Cultivation in Open Thin-Layer Cascade Photobioreactors with Water Recycling. Appl. Sci. 2020, 10, 3883. [Google Scholar] [CrossRef]
- Přibyl, P.; Cepák, V.; Zachleder, V. Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Appl. Microbiol. Biotechnol. 2012, 94, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Zienkiewicz, K.; Du, Z.-Y.; Ma, W.; Vollheyde, K.; Benning, C. Stress-induced neutral lipid biosynthesis in microalgae—Molecular, cellular and physiological insights. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2016, 1861, 1269–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, C.; Godfrey, V.; Wahlen, B.; Seefeldt, L.; Bugbee, B. Understanding precision nitrogen stress to optimize the growth and lipid content tradeoff in oleaginous green microalgae. Bioresour. Technol. 2013, 131, 188–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondioli, P.; Bella, L.D.; Rivolta, G.; Zittelli, G.C.; Bassi, N.; Rodolfi, L.; Casini, D.; Prussi, M.; Chiaramonti, D.; Tredici, M.R. Oil production by the marine microalgae Nannochloropsis sp. F&M-M24 and Tetraselmis suecica F&M-M33. Bioresour. Technol. 2012, 114, 567–572. [Google Scholar]
- Shakya, R.; Adhikari, S.; Mahadevan, R.; Hassan, E.B.; Dempster, T.A. Catalytic upgrading of bio-oil produced from hydrothermal liquefaction of Nannochloropsis sp. Bioresour. Technol. 2018, 252, 28–36. [Google Scholar] [CrossRef]
- Elliott, D.C. Review of recent reports on process technology for thermochemical conversion of whole algae to liquid fuels. Algal Res. 2016, 13, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Pedro, A.S.; González-López, C.V.; Acién, F.G.; Molina-Grima, E. Outdoor pilot production of Nannochloropsis gaditana: Influence of culture parameters and lipid production rates in raceway ponds. Algal Res. 2015, 8, 205–213. [Google Scholar] [CrossRef]
- Pedro, A.S.; González-López, C.V.; Acién, F.G.; Molina-Grima, E. Outdoor pilot-scale production of Nannochloropsis gaditana: Influence of culture parameters and lipid production rates in tubular photobioreactors. Bioresour. Technol. 2014, 169, 667–676. [Google Scholar] [CrossRef]
- Huntley, M.E.; Johnson, Z.I.; Brown, S.L.; Sills, D.L.; Gerber, L.; Archibald, I.; Machesky, S.C.; Granados, J.; Beal, C.; Greene, C.H. Demonstrated large-scale production of marine microalgae for fuels and feed. Algal Res. 2015, 10, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Darvehei, P.; Bahri, P.A.; Moheimani, N.R. Model development for the growth of microalgae: A review. Renew. Sustain. Energy Rev. 2018, 97, 233–258. [Google Scholar] [CrossRef]
- Lee, E.; Jalalizadeh, M.; Zhang, Q. Growth kinetic models for microalgae cultivation: A review. Algal Res. 2015, 12, 497–512. [Google Scholar] [CrossRef]







| Surface Area | 8 m2 | 50 m2 |
|---|---|---|
| Working volume | 55 L | 330 L |
| Surface-to-volume ratio | 145 m−1 | 152 m−1 |
| Volume flow | 2.4 L s−1 | 4.8 L s−1 |
| Suspension layer thickness | 0.6 cm | 0.6 cm |
| pH | 8.5 | 8.5 |
| # | Parameter | Value | Unit |
|---|---|---|---|
| 1 * | Extinction coefficient | 0.925 | L g−1 cm−1 |
| 2 a | Maximum biomass specific growth rate | 3.5 ± 1.1 | d−1 |
| 3 a | Light saturation constant | 633 ± 316 | µmol m−2 s−1 |
| 4 a | Photoinhibition constant | 1223 ± 60 | µmol m−2 s−1 |
| 5 a | Photoinhibition sensitivity factor | 12.6 ± 11.6 | - |
| 6 b | Urea saturation constant | 0.11 ± 0.04 | g L−1 |
| 7 * | Biomass yield coefficient with urea | 4.2 | g g−1 |
| 8 b | Nightly maximum biomass specific decay rate, | 0.17 ± 0.03 | d−1 |
| 9 c | Maximum biomass specific lipid formation rate | 0.08 ± 0.02 | g g−1 d−1 |
| 10 c | Maximum specific lipid-free growth rate | 0.54 ± 0.11 | g g−1 d−1 |
| 11 | Urea-limitation sensitivity factor | 2 | - |
| 12 * | Baseline lipid quota of M. salina | 0.15 | g g−1 |
| 13 * | Maximum lipid quota of M. salina | 0.46 | g g−1 |
| 14 c | Minimum nitrogen quota in lipid-free CDW | 0.06 ± 0.00 | g g−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schädler, T.; Thurn, A.-L.; Brück, T.; Weuster-Botz, D. Continuous Production of Lipids with Microchloropsis salina in Open Thin-Layer Cascade Photobioreactors on a Pilot Scale. Energies 2021, 14, 500. https://doi.org/10.3390/en14020500
Schädler T, Thurn A-L, Brück T, Weuster-Botz D. Continuous Production of Lipids with Microchloropsis salina in Open Thin-Layer Cascade Photobioreactors on a Pilot Scale. Energies. 2021; 14(2):500. https://doi.org/10.3390/en14020500
Chicago/Turabian StyleSchädler, Torben, Anna-Lena Thurn, Thomas Brück, and Dirk Weuster-Botz. 2021. "Continuous Production of Lipids with Microchloropsis salina in Open Thin-Layer Cascade Photobioreactors on a Pilot Scale" Energies 14, no. 2: 500. https://doi.org/10.3390/en14020500