Complexity of Electron Injection Dynamics and Light Soaking Effects in Efficient Dyes for Modern DSSC
Abstract
:1. Introduction
2. Materials and Methods
2.1. Device Fabrication
2.2. Device Characterization
3. Results and Discussion
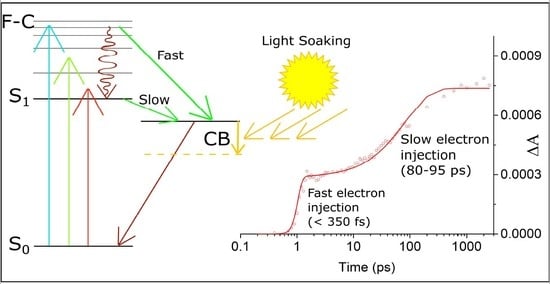
3.1. Monitoring Electron Injection in the Cells with Y123
3.2. Photovoltaic Performance and Continuous Irradiation Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar-cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High-Efficiency perovskite solar cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef] [PubMed]
- Chebrolu, V.T.; Kim, H.-J. Recent progress in quantum dot sensitized solar cells: An inclusive review of photoanode, sensitizer, electrolyte, and the counter electrode. J. Mater. Chem. 2019, 7, 4911–4933. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, J.; Wei, Q.; Zou, Y. Small-Molecule electron acceptors for efficient non-fullerene organic solar cells. Front. Chem. 2018, 6, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaels, H.; Rinderle, M.; Freitag, R.; Benesperi, I.; Edvinsson, T.; Socher, R.; Gagliardi, A.; Freitag, M. Dye-Sensitized solar cells under ambient light powering machine learning: Towards autonomous smart sensors for the internet of things. Chem. Sci. 2020, 11, 2895–2906. [Google Scholar] [CrossRef] [Green Version]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M.; et al. Dye-Sensitized solar cells for efficient power generation under ambient lighting. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Freitag, M.; Boschloo, G. The revival of dye-sensitized solar cells. Curr. Opin. Electrochem. 2017, 2, 111–119. [Google Scholar] [CrossRef]
- Koumura, N.; Wang, Z.S.; Mori, S.; Miyashita, M.; Suzuki, E.; Hara, K. Alkyl-Functionalized organic dyes for efficient molecular photovoltaics. J. Am. Chem. Soc. 2006, 128, 14256–14257. [Google Scholar] [CrossRef]
- Feldt, S.M.; Gibson, E.A.; Gabrielsson, E.; Sun, L.; Boschloo, G.; Hagfeldt, A. Design of organic dyes and cobalt polypyridine redox mediators for high-efficiency dye-sensitized solar cells. J. Am. Chem. Soc. 2010, 132, 16714–16724. [Google Scholar] [CrossRef]
- Ellis, H.; Eriksson, S.K.; Feldt, S.M.; Gabrielsson, E.; Lohse, P.W.; Lindblad, R.; Sun, L.; Rensmo, H.; Boschloo, G.; Hagfeldt, A. Linker unit modification of triphenylamine-based organic dyes for efficient cobalt mediated dye-sensitized solar cells. J. Phys. Chem. 2013, 117, 21029–21036. [Google Scholar] [CrossRef]
- Tsao, H.N.; Yi, C.; Moehl, T.; Yum, J.H.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Grätzel, M. Cyclopentadithiophene bridged donor-acceptor dyes achieve high power conversion efficiencies in dye-sensitized solar cells based on the tris-cobalt bipyridine redox couple. ChemSusChem 2011, 4, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Mueller, E.; Liska, P.; Vlachopoulos, N.; Graetzel, M. Conversion of light to electricity by cis-X2bis(2,2’-bipyridyl-4,4’-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on nanocrystalline titanium dioxide electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- Bai, Y.; Yu, Q.; Cai, N.; Wang, Y.; Zhang, M.; Wang, P. High-Efficiency organic dye-sensitized mesoscopic solar cells with a copper redox shuttle. Chem. Commun. 2011, 47, 4376–4378. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M.; Giordano, F.; Yang, W.; Pazoki, M.; Hao, Y.; Zietz, B.; Gra, M.; Hagfeldt, A.; Boschloo, G. Copper phenanthroline as a fast and high-performance redox mediator for dye-sensitized solar cells. J. Phys. Chem. 2016, 120, 9595–9603. [Google Scholar] [CrossRef]
- Saygili, Y.; Söderberg, M.; Pellet, N.; Giordano, F.; Cao, Y.; Munoz-García, A.B.; Zakeeruddin, S.M.; Vlachopoulos, N.; Pavone, M.; Boschloo, G.; et al. Copper bipyridyl redox mediators for dye-sensitized solar cells with high photovoltage. J. Am. Chem. Soc. 2016, 138, 15087–15096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.; Hanaya, M. Highly-Efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef]
- Ji, J.M.; Zhou, H.; Eom, Y.K.; Kim, C.H.; Kim, H.K. 14.2% efficiency dye-sensitized solar cells by co-sensitizing novel thieno[3,2-b]indole-based organic dyes with a promising porphyrin sensitizer. Adv. Energy Mater. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Direct contact of selective charge extraction layers enables high-efficiency molecular photovoltaics. Joule 2018, 2, 1108–1117. [Google Scholar] [CrossRef]
- Michaels, H.; Benesperi, I.; Edvinsson, T.; Muñoz-Garcia, A.B.; Pavone, M.; Boschloo, G.; Freitag, M. Copper complexes with tetradentate ligands for enhanced charge transport in dye-sensitized solar cells. Inorganics 2018, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Saygili, Y.; Ummadisingu, A.; Teuscher, J.; Luo, J.; Pellet, N.; Giordano, F.; Zakeeruddin, S.M.; Moser, J.-E.; Freitag, M.; et al. 11% efficiency solid-state dye-sensitized solar cells with copper(II/I) hole transport materials. Nat. Commun. 2017, 8, 15390. [Google Scholar] [CrossRef] [Green Version]
- Ghadiri, E.; Zakeeruddin, S.M.; Hagfeldt, A.; Gratzel, M.; Moser, J.E. Ultrafast charge separation dynamics in opaque, operational dye-sensitized solar cells revealed by femtosecond diffuse reflectance spectroscopy. Sci. Rep. 2016, 6, 24465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Z.; Guo, Y.; Wang, L.; Hao, Y.; Guo, Y.; Franchi, D.; Zhang, F.; Kloo, L.; Sun, L. Energy-Loss reduction as a strategy to improve the efficiency of dye-sensitized solar cells. Sol. RRL 2019, 3, 1900253. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Y.; Bahng, H.W.; Cao, Y.; Yi, C.; Saygili, Y.; Luo, J.; Liu, Y.; Kavan, L.; Moser, J.E.; et al. Comprehensive control of voltage loss enables 11.7% efficient solid-state dye-sensitized solar cells. Energy Environ. Sci. 2018, 11, 1779–1787. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Flores-Díaz, N.; Zhang, D.; Cao, Y.; Decoppet, J.D.; Fish, G.C.; Moser, J.E.; Zakeeruddin, S.M.; Wang, P.; Hagfeldt, A.; et al. Blue photosensitizer with copper(II/I) redox mediator for efficient and stable dye-sensitized solar cells. Adv. Funct. Mater. 2020, 30, 1–7. [Google Scholar] [CrossRef]
- Martín, C.; Ziółek, M.; Douhal, A. Ultrafast and fast charge separation processes in real dye-sensitizedsolar cells. J. Photochem. Photobiol. Photochem. Rev. 2016, 26, 1–30. [Google Scholar] [CrossRef]
- Gierszewski, M.; Grᶏdzka, I.; Glinka, A.; Ziółek, M. Insights into the limitations of solar cells sensitized with ruthenium dyes revealed in time-resolved spectroscopy studies. Phys. Chem. Chem. Phys. 2017, 19, 20463–20473. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, J.I. Interfacial charge-transfer transitions for direct charge-separation photovoltaics. Energies 2020, 13, 2521. [Google Scholar] [CrossRef]
- Fujisawa, J.I.; Hanaya, M. Light harvesting and direct electron injection by interfacial charge-transfer transitions between TiO2 and carboxy-anchor dye LEG4 in dye-sensitized solar cells. J. Phys. Chem. 2018, 122, 8–15. [Google Scholar] [CrossRef]
- Lindström, H.; Södergren, S.; Solbrand, A.; Rensmo, H.; Hjelm, J.; Hagfeldt, A.; Lindquist, S.-E. Li+ ion insertion in TiO2 (Anatase). 2. Voltammetry on nanoporous films. J. Phys. Chem. 1997, 101, 7717–7722. [Google Scholar] [CrossRef]
- Ellingson, J.R.; Asbury, J.B.; Ferrere, S.; Ghosh, H.N.; Sprague, J.R.; Lian, T.; Nozik, A.J. Sub-Picosecond injection of electrons from excited {Ru(2,2'-bipy-4,4'-dicarboxy)2(SCN)2} into TiO2 using transient mid-infrared spectroscopy. In Proceedings of the at the 12th International Conference onPhotochemical Conversion and Storage of Solar Energy, Berlin, Germany, 9–14 August 1998. [Google Scholar]
- Hilgendorff, M.; Sundström, V. Dynamics of electron injection and recombination of dye-sensitized TiO2 particles. J. Phys. Chem. 1998, 102, 10505–10514. [Google Scholar] [CrossRef]
- Durrant, J.R.; Haque, S.A.; Palomares, E. Towards optimisation of electron transfer processes in dye sensitised solar cells. Coord. Chem. Rev. 2004, 248, 1247–1257. [Google Scholar] [CrossRef]
- Andersen, N.A.; Lian, T. Ultrafast electron transfer at the molecule-semiconductor nanoparticle interface. Annu. Rev. Phys. Chem. 2005, 56, 491–519. [Google Scholar] [CrossRef] [PubMed]
- Ardo, S.; Meyer, G.J. Photodriven heterogeneous charge transfer with transition-metal compounds anchored to TiO2 semiconductor surfaces. Chem. Soc. Rev. 2009, 38, 115–164. [Google Scholar] [CrossRef] [PubMed]
- Katoh, R.; Furube, A. Electron injection efficiency in dye-sensitized solar cells. J. Photochem. Photobiol. Photochem. Rev. 2014, 20, 1–16. [Google Scholar] [CrossRef]
- Ponseca, C.S.; Chábera, P.; Uhlig, J.; Persson, P.; Sundström, V. Ultrafast electron dynamics in solar energy conversion. Chem. Rev. 2017, 117, 10940–11024. [Google Scholar] [CrossRef]
- Listorti, A.; Creager, C.; Sommeling, P.; Kroon, J.; Palomares, E.; Fornelli, A.; Breen, B.; Barnes, P.R.F.; Durrant, J.R.; Law, C.; et al. The mechanism behind the beneficial effect of light soaking on injection efficiency and photocurrent in dye sensitized solar cells. Energy Environ. Sci. 2011, 4, 3494–3501. [Google Scholar] [CrossRef] [Green Version]
- Cabau, L.; Pellejà, L.; Clifford, J.N.; Kumar, C.V.; Palomares, E. Light soaking effects on charge recombination and device performance in dye sensitized solar cells based on indoline-cyclopentadithiophene chromophores. J. Mater. Chem. 2013, 1, 8994–9000. [Google Scholar] [CrossRef]
- Gao, J.; El-zohry, A.M.; Trilaksana, H.; Gabrielsson, E.; Leandri, V.; Ellis, H.; Amario, L.D.; Safdari, M.; Gardner, J.M.; Andersson, G.; et al. Light-Induced interfacial dynamics dramatically improve the photocurrent in dye-sensitized solar cells: An electrolyte effect. ACS Appl. Mater. Interfaces 2018, 10, 26241–26247. [Google Scholar] [CrossRef]
- Gao, J.; Yang, W.; El-Zohry, A.M.; Prajapati, G.K.; Fang, Y.; Dai, J.; Hao, Y.; Leandri, V.; Svensson, P.H.; Furó, I.; et al. Light-Induced electrolyte improvement in cobalt tris(bipyridine)-mediated dye-sensitized solar cells. J. Mater. Chem. 2019, 7, 19495–19505. [Google Scholar] [CrossRef] [Green Version]
- Sobuś, J.; Kubicki, J.; Burdziński, G.; Ziołek, M. Carbazole dye-sensitized solar cells studied from femtoseconds to seconds—effect of additives in cobalt- and iodide-based electrolytes. ChemSusChem 2015, 8, 3118–3128. [Google Scholar] [CrossRef]
- Sobuś, J.; Gierczyk, B.; Burdziński, G.; Jancelewicz, M.; Polanski, E.; Hagfeldt, A.; Ziółek, M. Factors affecting the performance of champion silyl-anchor carbazole dye revealed in the femtosecond to second studies of complete ADEKA-1 sensitized solar cells. Chem. Eur. J. 2016, 22, 15807–15818. [Google Scholar] [CrossRef] [PubMed]
- Gierszewski, M.; Glinka, A.; Gradzka, I.; Jancelewicz, M.; Ziółek, M. Effects of post-assembly molecular and atomic passivation of sensitized titania surface: Dynamics of electron transfer measured from femtoseconds to seconds. ACS Appl. Mater. Interfaces 2017, 9, 17102–17114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glinka, A.; Gierszewski, M.; Ziółek, M. Effects of aqueous electrolyte, active layer thickness and bias irradiation on charge transfer rates in solar cells sensitized with top efficient carbazole dyes. J. Phys. Chem. 2018, 122, 8147–8158. [Google Scholar] [CrossRef]
- Pydzińska-Białek, K.; Glinka, A.; Drushliak, V.; Nowaczyk, G.; Florczak, P.; Ziółek, M. Impact of improvements in mesoporous titania layers on ultrafast electron transfer dynamics in perovskite and dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2020, 22, 21947–21960. [Google Scholar] [CrossRef] [PubMed]
- Glinka, A.; Gierszewski, M.; Gierczyk, B.; Burdziński, G.; Michaels, H.; Freitag, M.; Ziółek, M. Interface modification and exceptionally fast regeneration in copper mediated solar cells sensitized with indoline dyes. J. Phys. Chem. 2020, 124, 2895–2906. [Google Scholar] [CrossRef]
- Idígoras, J.; Burdziński, G.; Karolczak, J.; Kubicki, J.; Oskam, G.; Anta, J.A.; Ziółek, M. The impact of the electrical nature of the metal oxide on the performance in dye-sensitized solar cells: New look at old paradigms. J. Phys. Chem. 2015, 119, 3931–3944. [Google Scholar] [CrossRef]
- Haque, S.A.; Tachibana, Y.; Willis, R.L.; Moser, J.E.; Grätzel, M.; Klug, D.R.; Durrant, J.R. Parameters influencing charge recombination kinetics in dye-sensitized nanocrystalline titanium dioxide films. J. Phys. Chem. 2000, 104, 538–547. [Google Scholar] [CrossRef] [Green Version]
- Nelson, J.; Haque, S.A.; Klug, D.R.; Durrant, J.R. Trap-Limited recombination in dye-sensitized nanocrystalline metal oxide electrodes. Phys. Rev. 2001, 63, 205321. [Google Scholar] [CrossRef]
- Xiang, W.; Huang, W.; Bach, U.; Spiccia, L. Stable high efficiency dye-sensitized solar cells based on a cobalt polymer gel electrolyte. Chem. Commun. 2013, 49, 8997–8999. [Google Scholar] [CrossRef]
- Wang, Z.S.; Koumura, N.; Cui, Y.; Takahashi, M.; Sekiguchi, H.; Mori, A.; Kubo, T.; Furube, A.; Hara, K. Hexylthiophene-Functionalized carbazole dyes for efficient molecular photovoltaics: Tuning of solar-cell performance by structural modification. Chem. Mater. 2008, 20, 3993–4003. [Google Scholar] [CrossRef]
- Gierszewski, M.; Glinka, A.; Grądzka, I.; Gierczyk, B.; Ziółek, M. Testing new concepts in solar cells sensitized with indoline dyes—Alkoxysilyl anchoring group, molecular capping, and cobalt-based electrolyte. J. Phys. Chem. 2018, 122, 25764–25775. [Google Scholar] [CrossRef]





| Cell | PCE [%] | Voc [V] | FF | Jsc [mA cm−2] | Total APCE |
|---|---|---|---|---|---|
| Y123 Co3+/2+(bpy)3 | 3.8 | 0.96 | 0.57 | 7.03 | 0.69 |
| error | 0.2 | 0.01 | 0.02 | 0.15 | 0.02 |
| Y123 Cu2+/+(tmby)2 | 4.7 | 1.06 | 0.60 | 7.47 | 0.73 |
| error | 0.2 | 0.01 | 0.01 | 0.06 | 0.01 |
| MK2 Co3+/2+(bpy)3 | 5.1 | 0.85 | 0.65 | 9.22 | 0.71 |
| error | 0.2 | 0.01 | 0.02 | 0.11 | 0.01 |
| Cell | PCE Ratio | Voc Ratio | FF Ratio | Jsc Ratio |
|---|---|---|---|---|
| Y123 Co3+/2+(bpy)3 | 0.99 | 0.96 | 0.98 | 1.04 |
| Y123 Cu2+/+(tmby)2 | 0.81 | 0.96 | 0.82 | 1.03 |
| MK2 Co3+/2+(bpy)3 | 1.04 | 0.96 | 1.03 | 1.04 |
| Fresh | Irradiated | |||||
|---|---|---|---|---|---|---|
| τ1 [ps] | τ2 [ps] | τ3 [ps] | τ1 [ps] | τ2 [ps] | τ3 [ps] | |
| Y123 Co3+/2+(bpy)3 a | 1.0 | 18 | 370 | 0.7 | 13 | 310 |
| Y123 Cu2+/+(tmby)2 a | 1.5 | 12 | 530 | 1.4 | 10 | 420 |
| MK2 Co3+/2+(bpy)3 b | 4.1 | 35 | 380 | 2.9 | 31 | 400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glinka, A.; Kubicki, J.; Ziółek, M. Complexity of Electron Injection Dynamics and Light Soaking Effects in Efficient Dyes for Modern DSSC. Energies 2021, 14, 407. https://doi.org/10.3390/en14020407
Glinka A, Kubicki J, Ziółek M. Complexity of Electron Injection Dynamics and Light Soaking Effects in Efficient Dyes for Modern DSSC. Energies. 2021; 14(2):407. https://doi.org/10.3390/en14020407
Chicago/Turabian StyleGlinka, Adam, Jacek Kubicki, and Marcin Ziółek. 2021. "Complexity of Electron Injection Dynamics and Light Soaking Effects in Efficient Dyes for Modern DSSC" Energies 14, no. 2: 407. https://doi.org/10.3390/en14020407