High-Temperature Fluidized Bed Processing of Waste Electrical and Electronic Equipment (WEEE) as a Way to Recover Raw Materials
Abstract
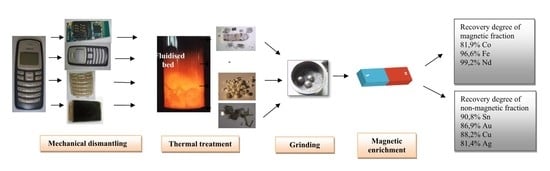
:1. Introduction
2. Materials and Methods
2.1. The Thermal Treatment Process
2.2. Magnetic Enrichment
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MPhW | mobile phone waste |
| PCA | printed circuit assembly |
| PCBA | printed circuit board assembly |
| VOC | volatile organic compound |
| WEEE | waste electrical and electronic equipment |
| 0 | raw material after MPhW thermal treatment |
| I | magnetic fraction (>1 mm) after magnetic separation by neodymium magnet |
| II | non-magnetic fraction (>1 mm) after magnetic separation by neodymium magnet |
| III | magnetic fraction (<1 mm) after enrichment by three-phase plate electromagnetic separator |
| IV | magnetic fraction (<1 mm) after enrichment by belt type 1-disc electromagnetic separator |
| V | magnetic fraction (<0.5 mm) after enrichment by belt type 1-disc electromagnetic separator |
| VI | non-magnetic fraction (<0.5 mm) after enrichment by belt type 1-disc electromagnetic separator |
| Greek symbols | |
| γ | yield of the fraction; kgi/kg0, i {I,II,III,IV,V,VI} |
| ∑γ | the accumulated yield of the fractions; kg/kg0 |
| λ | the metal content in the fraction; kgMe,i/kgi |
| α | the metal content in the feed; kgMe,0/kg0 |
| β | the metal content in the combined fractions; kgMe/kg |
| K | enrichment factor |
| ε | yield of the metal; kgMe,i/kg0 |
| ∑ ε | the accumulated yield of the metal; kgMe/kg0 |
References
- Maragkos, K.G.; Hahladakis, J.N.; Gidarkos, E. Qualitative and quantitative determination of heavy metals in waste cellular phones. Waste Manag. 2013, 33, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Balde, C.P.; Kuehr, R.; Blumenthal, K.; Gill, S.F.; Kern, M.; Micheli, P.; Magpantay, E.; Huisman, J. E-Waste Statistics: Guidelines on Classifications, Reporting and Indicators; United Nations University: Bonn, Germany, 2015; ISBN 978-92-8-084554-9. [Google Scholar]
- Khetriwal, D.S.; Kraeuchi, P.; Widmer, R. Producer responsibility for e-waste management: Key issues for consideration e learning from the Swiss experience. J. Environ. Manag. 2009, 90, 153–165. [Google Scholar] [CrossRef]
- Liu, W.; Chen, L.; Tian, J. Uncovering the Evolution of Lead In-Use Stocks in Lead-Acid Batteries and the Impact on Future Lead Metabolism in China. Environ. Sci. Technol. 2016, 50, 5412–5419. [Google Scholar] [CrossRef] [PubMed]
- Tanskanen, P. Management and recycling of electronic waste. Acta Mater. 2013, 61, 1001–1011. [Google Scholar] [CrossRef]
- Tuncuk, A.; Stazi, V.; Akcil, A.; Yazici, E.Y.; Deveci, H. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Bachér, J.; Mrotzek, A.; Wahlström, M. Mechanical pre-treatment of mobile phones and its effect on the Printed Circuit Assemblies (PCAs). Waste Manag. 2015, 45, 235–245. [Google Scholar] [CrossRef]
- Frost and Sullivan. European Waste Electrical and Electronics Equipment Recycling Market. 2013. Available online: http://www.endseurope.com/docs/131001c.pdf (accessed on 18 September 2020).
- Boldoczki, S.; Thorenz, A.; Tuma, A. The environmental impacts of preparation for reuse: A case study of WEEE reuse in Germany. J. Clean. Prod. 2019, 252, 119736. [Google Scholar] [CrossRef]
- Baldé, C.P.; Wang, F.; Kuehr, R.; Huisman, J. The Global e-Waste Monitor—2014; United Nations University, IAS–SCYCLE: Bonn, Germany, 2015. [Google Scholar]
- Batteiger, A.; Rotter, V.S. Material Implications of Rural Electrification—A Methodological Framework to Assess In-Use Stocks of Off-Grid Solar Products and EEE in Rural Households in Bangladesh. Recycling 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Robinson, B.H. E-waste: An assessment of global production and environmental impacts. Sci. Total Environ. 2009, 408, 183–191. [Google Scholar] [CrossRef] [PubMed]
- UNEP. Call for Global Action on E-Waste—United Nations Environment Programme (UNEP). 2013. Available online: http://www.unep.org/Documents.Multilingual/Default.asp?ArticleID=5447&DocumentID=496&l=en (accessed on 20 August 2020).
- Glöser, S.; Soulier, M.; Espinoza, L.A.T. Dynamic Analysis of Global Copper Flows. Global Stocks, Postconsumer Material Flows, Recycling Indicators, and Uncertainty Evaluation. Environ. Sci. Technol. 2013, 47, 6564–6572. [Google Scholar] [CrossRef]
- Nikulski, J.; Ritthoff, M.; von Gries, N. The Potential and Limitations of Critical Raw Material Recycling: The Case of LED Lamps. Resources 2021, 10, 37. [Google Scholar] [CrossRef]
- Oguchi, M.; Sakanakura, H.; Terazono, A.; Takigami, H. Fate of metals contained in waste electrical and electronic equipment in a municipal waste treatment process. Waste Manag. 2012, 32, 96–103. [Google Scholar] [CrossRef]
- Kasper, A.C.; Bernardes, A.M.; Veit, H.M. Characterization and recovery of polymers from mobile phone scrap. Waste Manag. Res. 2011, 29, 714–726. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, G. A novel hybrid MCDM framework for WEEE recycling partner evaluation on the basis of green competencies. J. Clean. Prod. 2019, 241, 118017. [Google Scholar] [CrossRef]
- Directive 2002/96/EC of the European Parliament and of the Council of 27 January 2003 on Waste Electrical and Electronic Equipment. Available online: https://lexparency.org/eu/32002L0096/ (accessed on 4 July 2021).
- Directive 2012/19/EU of the European Parliament and of the Council of 4 July 2012 on Waste Electrical and Electronic Equipment (WEEE). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012L0019&from=EN (accessed on 4 July 2021).
- Cui, J.; Forssberg, E. Mechanical recycling of waste electric and electronic equipment: A review. J. Hazard. Mater. 2003, 99, 243–263. [Google Scholar] [CrossRef]
- Rao, S.R. Recycling Metal. In Waste Management Series; Rao, S.R., Ed.; Vol. 7–Resources Recovery and Recycling from Metallurgical Wastes; Elsevier Science: Amsterdam, The Netherlands, 2006; Chapter 7. [Google Scholar]
- Xu, C.; Zhang, W.; He, W.; Li, G.; Huang, J. The situation of waste mobile phone management in developed countries and development status in China. Waste Manag. 2016, 58, 341–347. [Google Scholar] [CrossRef]
- Eurostat. Waste Statistics–Electrical and Electronic Equipment. 2016. Available online: http://ec.europa.eu/eurostat/statisticsexplained/index.php/Waste_statistics_electrical_and_electronic_equipment#EEE_put_on_the_market_and_WEEE_collected_and_treated_in_the_EU (accessed on 27 January 2020).
- Ismail, H.; Hanafiah, M.M. An overview of LCA application in WEEE management: Current practices, progress and challenges. J. Clean. Prod. 2019, 232, 79–93. [Google Scholar] [CrossRef]
- Khaliq, A.; Rhamdhani, M.A.; Brooks, G.; Masood, S. Metal Extraction Processes for Electronic Waste and Existing Industrial Routes: A Review and Australian Perspective. Resources 2014, 3, 152–179. [Google Scholar] [CrossRef] [Green Version]
- Kiddee, P.; Naidu, R.; Wong, M.H. Electronic waste management approaches: An overview. Waste Manag. 2013, 33, 1237–1250. [Google Scholar] [CrossRef]
- Kolias, K.; Hahladakis, J.N.; Gidarakos, E. Assessment of toxic metals in waste personal computers. Waste Manag. 2014, 34, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Salhofer, S.; Steuer, B.; Ramusch, R.; Beigl, P. WEEE management in Europe and China–A comparison. Waste Manag. 2016, 57, 27–35. [Google Scholar] [CrossRef]
- Singh, N.; Duan, H.; Yin, F.; Song, Q.; Li, J. Characterizing the Materials Composition and Recovery Potential from Waste Mobile Phones: A Comparative Evaluation of Cellular and Smart Phones. ACS Sustain. Chem. Eng. 2018, 6, 13016–13024. [Google Scholar] [CrossRef]
- Widmer, R.; Oswald-Krapf, H.; Sinha-Khetriwal, D.; Schnellmann, M.; Böni, H. Global perspectives on e-waste. Environ. Impact Assess. Rev. 2005, 25, 436–458. [Google Scholar] [CrossRef]
- Kasper, A.C.; Berselli, G.B.T.; Freitas, B.D.; Tenório, J.A.S.; Bernardes, A.M. Printed wiring boards from mobile phones: Characterization and recycling of copper. Waste Manag. 2011, 31, 2536–2545. [Google Scholar] [CrossRef]
- Monneron-Enaud, B.; Wiche, O.; Schlömann, M. Biodismantling, a Novel Application of Bioleaching in Recycling of Electronic Wastes. Recycling 2020, 5, 22. [Google Scholar] [CrossRef]
- Murugan, R.V.; Bharat, S.; Deshpande, A.P.; Varughese, S.; Haridoss, P. Milling and separation of the multi-component printed circuit board materials and the analysis of elutriation based on a single particle model. Powder Technol. 2008, 183, 169–176. [Google Scholar] [CrossRef]
- Palmieri, R.; Bonifazi, G.; Serranti, S. Recycling-oriented characterization of plastic frames and printed circuit boards from mobile phones by electronic and chemical imaging. Waste Manag. 2014, 34, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Yamane, L.H.; de Moraes, V.T.; Espinosa, D.C.R.; Ten’orio, J. Recycling of WEEE: Characterization of spent printed circuit boards from mobile phones and computers. Waste Manag. 2011, 31, 2553–2558. [Google Scholar] [CrossRef]
- Kaya, M. Electronic Waste and Printed Circuit Board Recycling Technologies; Springer: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Rene, E.R.; Sethurajan, M.; Ponnusamy, V.K.; Kumar, G.; Dung, T.N.B.; Brindhadevi, K.; Pugazhendhi, A. Electronic waste generation, recycling and resource recovery: Technological perspectives and trends. J. Hazard. Mater. 2021, 416, 125664. [Google Scholar] [CrossRef]
- Rieger, T.; Oey, J.; Palchyk, V.; Hofmann, A.; Franke, M.; Hornung, A. Chemical Recycling of WEEE Plastics—Production of High Purity Monocyclic Aromatic Chemicals. Processes 2021, 9, 530. [Google Scholar] [CrossRef]
- Woynarowska, A.; Żelazny, S.; Żukowski, W. Thermal treatment of electronic waste in a fluidised bed and chemical digestion of solid products. Waste Manag. Res. 2016, 34, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Szałkowski, K.; Szafran, M.; Bobryk, E.; Sentek, J. Chemical Technology-Inorganic Industry; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2013. [Google Scholar]
- Cesaro, A.; Belgiorno, V.; Gorrasi, G.; Viscusi, G.; Vaccari, M.; Vinti, G.; Jandric, A.; Dias, M.I.; Hursthouse, A.; Salhofer, S. A relative risk assessment of the open burning of WEEE. Environ. Sci. Pollut. Res. 2019, 26, 11042–11052. [Google Scholar] [CrossRef] [Green Version]
- Berkowicz, G.; Majka, T.; Żukowski, W. The pyrolysis and combustion of polyoxymethylene in a fluidised bed with the possibility of incorporating CO2. Energy Convers. Manag. 2020, 214, 112888. [Google Scholar] [CrossRef]
- Żukowski, W.; Berkowicz, G. The combustion of polyolefins in inert and catalytic fluidised beds. J. Clean. Prod. 2019, 236, 117663. [Google Scholar] [CrossRef]
- Żukowski, W.; Berkowicz, G. The combustion of liquids and low-density solids in a cenospheric fluidised bed. Combust. Flame 2019, 206, 476–489. [Google Scholar] [CrossRef]
- Żukowski, W.; Berkowicz, G. Hydrogen production through the partial oxidation of methanol using N2O in a fluidised bed of an iron-chromium catalyst. Int. J. Hydrogen Energy 2017, 42, 28247–28253. [Google Scholar] [CrossRef]
- Olek, M.; Baron, J.; Żukowski, W. Thermal decomposition of selected chlorinated hydrocarbons during gas combustion in fluidized bed. Chem. Cent. J. 2013, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Olek, M.; Kandefer, S.; Kaniowski, W.; Żukowski, W.; Baron, J. Carbon shale combustion in the fluidized bed reactor. Pol. J. Chem. Technol. 2014, 16. [Google Scholar] [CrossRef] [Green Version]
- Żukowski, W.; Jankowski, D.; Baron, J.; Wrona, J. Combustion dynamics of polymer wastes in a bubbling fluidized bed. J. Clean. Prod. 2021, 320, 128807. [Google Scholar] [CrossRef]




| Mobile Phone before Mechanical Dismantling | Mobile Phone after Mechanical Dismantling |
|---|---|
 |  |
| kit no. I | |
 |  |
| kit no. II | |
 |  |
| kit no. III | |
| No. | Sample before Thermal Treatment | m0 (g) | Sample after Thermal Treatment | m1 (g) | Weight Loss (%) |
|---|---|---|---|---|---|
| 1 |  | 2.68 |  | 1.86 | 30.60 |
| 2 |  | 4.24 |  | 2.82 | 33.50 |
| 3 |  | 0.96 |  | 0.30 | 68.75 |
| 4 |  | 4.95 |  | 3.24 | 34.54 |
| 5 |  | 3.15 |  | 2.39 | 24.13 |
| 6 |  | 3.06 | - | 0.00 | 100 |
| 7 |  | 2.18 |  | 1.95 | 10.55 |
| 8 |  | 3.46 |  | 2.13 | 38.44 |
| 9 |  | 2.30 | - | 0.00 | 100 |
| 10 |  | 3.54 |  | 2.72 | 23.16 |
| 11 |  | 2.35 |  | 0.64 | 72.77 |
| No. | Sample before Thermal Treatment | m0 (g) | Sample after Thermal Treatment | m1 (g) | Weight Loss (%) |
|---|---|---|---|---|---|
| 12 |  | 4.13 |  | 2.78 | 32.69 |
| 13 |  | 2.20 |  | 0.58 | 73.63 |
| 14 |  | 3.29 |  | 2.38 | 27.66 |
| 15 |  | 1.90 |  | 1.71 | 10.00 |
| 16 |  | 4.69 |  | 3.54 | 24.52 |
| 17 |  | 4.81 |  | 3.40 | 29.31 |
| 18 |  | 2.14 | - | 0.00 | 100 |
| 19 |  | 4.73 |  | 4.41 | 6.76 |
| 20 |  | 6.00 |  | 4.84 | 19.33 |
| No. | Mass (g) | Emission (mg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | CO | C6H6 | C6H6O | C10H8 | CH4 | C2H6 | C2H2 | C2H4 | VOCs | |
| 1 | 2.68 | 235 | 4.54 | 0.69 | 1.54 | 1.61 | 0.62 | 0.87 | 1.59 | 11.46 |
| 2 | 4.24 | 317 | 5.71 | 1.25 | 2.49 | 2.31 | 0.99 | 1.02 | 1.99 | 15.76 |
| 3 | 0.96 | 307 | 4.95 | 0.57 | 2.19 | 2.30 | 0.88 | 1.08 | 2.92 | 14.89 |
| 4 | 4.95 | 397 | 7.30 | 2.04 | 3.25 | 3.09 | 1.32 | 1.13 | 2.60 | 20.73 |
| 5 | 3.16 | 290 | 5.26 | 0.27 | 4.07 | 2.52 | 1.00 | 1.01 | 2.81 | 16.94 |
| 6 | 3.06 | 724 | 23.5 | 13.4 | 10.4 | 5.05 | 4.40 | 2.22 | 3.00 | 61.97 |
| 7 | 2.18 | 14.0 | 0.32 | 0.10 | 0.17 | 0.05 | 0.00 | 0.00 | 0.04 | 0.68 |
| 8 | 3.46 | 309 | 5.35 | 2.42 | 2.00 | 3.11 | 1.06 | 0.47 | 1.50 | 15.91 |
| 9 | 2.30 | 711 | 20.3 | 10.6 | 9.67 | 4.75 | 3.44 | 2.21 | 3.65 | 54.62 |
| 10 | 3.54 | 327 | 6.70 | 4.48 | 2.32 | 3.84 | 1.57 | 0.45 | 1.66 | 21.02 |
| 11 | 2.35 | 255 | 1.41 | 0.03 | 0.25 | 3.52 | 0.32 | 0.36 | 1.15 | 7.04 |
| 12 | 4.13 | 463 | 8.13 | 4.65 | 3.20 | 4.34 | 1.85 | 0.70 | 2.29 | 25.16 |
| 13 | 2.21 | 240 | 3.59 | 0.53 | 1.13 | 2.16 | 0.54 | 1.11 | 1.89 | 10.95 |
| 14 | 3.29 | 374 | 5.30 | 2.11 | 2.46 | 3.63 | 1.09 | 0.53 | 1.94 | 17.06 |
| 15 | 1.90 | 242 | 5.98 | 1.54 | 1.83 | 2.62 | 0.85 | 0.51 | 1.65 | 14.98 |
| 16 | 4.69 | 232 | 3.82 | 0.32 | 1.02 | 2.11 | 0.67 | 0.94 | 2.46 | 11.34 |
| 17 | 4.81 | 534 | 5.91 | 1.62 | 2.53 | 3.81 | 1.09 | 0.88 | 2.50 | 18.34 |
| 18 | 2.14 | 750 | 20.6 | 2.60 | 10.4 | 3.79 | 2.32 | 3.26 | 4.55 | 47.52 |
| 19 | 4.73 | 682 | 7.31 | 2.61 | 3.23 | 5.29 | 1.71 | 1.04 | 3.76 | 24.95 |
| 20 | 6.00 | 692 | 9.55 | 3.34 | 4.41 | 5.85 | 2.21 | 0.94 | 3.95 | 30.25 |
| ∑ | 66.76 | 8093 | 156 | 55.2 | 69.0 | 66.0 | 27.9 | 20.7 | 47.9 | 442.7 |
| No. | Mass (g) | Emission Coefficient (mg g−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | CO | C6H6 | C6H6O | C10H8 | CH4 | C2H6 | C2H2 | C2H4 | VOCs | |
| 1 | 2.68 | 87.9 | 1.70 | 0.26 | 0.58 | 0.60 | 0.23 | 0.32 | 0.60 | 4.29 |
| 2 | 4.24 | 74.8 | 1.35 | 0.29 | 0.59 | 0.55 | 0.23 | 0.24 | 0.47 | 3.72 |
| 3 | 0.96 | 319 | 5.15 | 0.60 | 2.28 | 2.39 | 0.91 | 1.13 | 3.03 | 15.5 |
| 4 | 4.95 | 80.2 | 1.48 | 0.41 | 0.66 | 0.63 | 0.27 | 0.23 | 0.53 | 4.21 |
| 5 | 3.16 | 91.9 | 1.67 | 0.09 | 1.29 | 0.80 | 0.32 | 0.32 | 0.89 | 5.38 |
| 6 | 3.06 | 236 | 7.68 | 4.39 | 3.40 | 1.65 | 1.44 | 0.72 | 0.98 | 20.3 |
| 7 | 2.18 | 6.41 | 0.15 | 0.05 | 0.08 | 0.02 | 0.00 | 0.00 | 0.02 | 0.32 |
| 8 | 3.46 | 89.2 | 1.54 | 0.70 | 0.58 | 0.9 | 0.31 | 0.14 | 0.43 | 4.60 |
| 9 | 2.30 | 309 | 8.82 | 4.60 | 4.20 | 2.07 | 1.49 | 0.96 | 1.59 | 23.7 |
| 10 | 3.54 | 92.3 | 1.89 | 1.26 | 0.65 | 1.09 | 0.44 | 0.13 | 0.47 | 5.93 |
| 11 | 2.35 | 109 | 0.60 | 0.00 | 0.11 | 1.5 | 0.14 | 0.15 | 0.49 | 2.99 |
| 12 | 4.13 | 112 | 1.97 | 1.12 | 0.77 | 1.05 | 0.45 | 0.17 | 0.55 | 6.08 |
| 13 | 2.21 | 109 | 1.63 | 0.24 | 0.51 | 0.98 | 0.24 | 0.50 | 0.86 | 4.96 |
| 14 | 3.29 | 114 | 1.61 | 0.64 | 0.75 | 1.11 | 0.33 | 0.16 | 0.59 | 5.19 |
| 15 | 1.90 | 127 | 3.14 | 0.81 | 0.96 | 1.38 | 0.45 | 0.27 | 0.87 | 7.88 |
| 16 | 4.69 | 49.4 | 0.81 | 0.07 | 0.22 | 0.45 | 0.14 | 0.20 | 0.53 | 2.42 |
| 17 | 4.81 | 111 | 1.23 | 0.34 | 0.53 | 0.79 | 0.23 | 0.18 | 0.52 | 3.82 |
| 18 | 2.14 | 351 | 9.64 | 1.22 | 4.85 | 1.77 | 1.08 | 1.52 | 2.13 | 22.2 |
| 19 | 4.73 | 144 | 1.55 | 0.55 | 0.68 | 1.12 | 0.36 | 0.22 | 0.79 | 5.27 |
| 20 | 6.00 | 115 | 1.59 | 0.56 | 0.73 | 0.97 | 0.37 | 0.16 | 0.66 | 5.04 |
| Average coefficient | 136 | 2.76 | 0.91 | 1.22 | 1.09 | 0.47 | 0.39 | 0.85 | 7.69 | |
| No. | Mass (g) | Emission (mg) | |||||
|---|---|---|---|---|---|---|---|
| Sample | SO2 | NOx | HCN | NH3 | HBr | HCl | |
| 1 | 2.68 | 2.06 | 3.39 | 1.15 | 0.00 | 0.00 | 0.00 |
| 2 | 4.24 | 1.82 | 3.20 | 0.84 | 0.00 | 0.00 | 0.00 |
| 3 | 0.96 | 2.41 | 1.64 | 1.16 | 0.00 | 0.00 | 0.00 |
| 4 | 4.95 | 1.44 | 3.91 | 1.36 | 0.00 | 0.00 | 0.00 |
| 5 | 3.16 | 3.31 | 0.74 | 0.70 | 0.00 | 0.00 | 0.00 |
| 6 | 3.06 | 3.03 | 1.25 | 1.83 | 0.00 | 2.39 | 0.00 |
| 7 | 2.18 | 0.07 | 4.67 | 0.00 | 0.00 | 0.00 | 0.00 |
| 8 | 3.46 | 2.92 | 3.45 | 1.02 | 0.12 | 0.00 | 2.58 |
| 9 | 2.30 | 4.95 | 0.42 | 1.16 | 0.00 | 0.00 | 0.00 |
| 10 | 3.54 | 3.10 | 3.73 | 0.98 | 0.24 | 0.00 | 3.45 |
| 11 | 2.35 | 0.02 | 0.02 | 0.34 | 0.00 | 0.00 | 0.00 |
| 12 | 4.13 | 4.59 | 4.90 | 1.42 | 0.25 | 0.00 | 3.33 |
| 13 | 2.21 | 2.46 | 0.46 | 0.65 | 0.00 | 0.00 | 0.00 |
| 14 | 3.29 | 3.12 | 4.09 | 1.15 | 0.13 | 0.00 | 2.03 |
| 15 | 1.90 | 1.47 | 1.96 | 0.86 | 0.00 | 0.00 | 1.27 |
| 16 | 4.69 | 1.61 | 0.62 | 0.54 | 0.00 | 0.00 | 0.00 |
| 17 | 4.81 | 3.81 | 4.91 | 1.19 | 0.08 | 0.00 | 1.86 |
| 18 | 2.14 | 6.00 | 6.13 | 3.41 | 0.00 | 0.00 | 0.00 |
| 19 | 4.73 | 5.6 | 7.73 | 1.72 | 0.18 | 2.62 | 2.8 |
| 20 | 6.00 | 5.51 | 5.13 | 1.21 | 0.14 | 4.43 | 2.46 |
| ∑ | 66.76 | 59.3 | 62.4 | 22.7 | 1.15 | 9.44 | 19.8 |
| No. | Mass (g) | Emission Coefficient (mg g−1) | |||||
|---|---|---|---|---|---|---|---|
| Sample | SO2 | NOx | HCN | NH3 | HBr | HCl | |
| 1 | 2.68 | 0.77 | 1.27 | 0.43 | 0.00 | 0.00 | 0.00 |
| 2 | 4.24 | 0.43 | 0.76 | 0.20 | 0.00 | 0.00 | 0.00 |
| 3 | 0.96 | 2.51 | 1.71 | 1.21 | 0.00 | 0.00 | 0.00 |
| 4 | 4.95 | 0.29 | 0.79 | 0.28 | 0.00 | 0.00 | 0.00 |
| 5 | 3.16 | 1.05 | 0.23 | 0.22 | 0.00 | 0.00 | 0.00 |
| 6 | 3.06 | 0.99 | 0.41 | 0.60 | 0.00 | 0.78 | 0.00 |
| 7 | 2.18 | 0.03 | 2.14 | 0.00 | 0.00 | 0.00 | 0.00 |
| 8 | 3.46 | 0.84 | 1.00 | 0.29 | 0.04 | 0.00 | 0.74 |
| 9 | 2.30 | 2.15 | 0.18 | 0.50 | 0.00 | 0.00 | 0.00 |
| 10 | 3.54 | 0.88 | 1.05 | 0.28 | 0.07 | 0.00 | 0.97 |
| 11 | 2.35 | 0.01 | 0.01 | 0.14 | 0.00 | 0.00 | 0.00 |
| 12 | 4.13 | 1.11 | 1.19 | 0.34 | 0.06 | 0.00 | 0.81 |
| 13 | 2.21 | 1.12 | 0.21 | 0.29 | 0.00 | 0.00 | 0.00 |
| 14 | 3.29 | 0.95 | 1.24 | 0.35 | 0.04 | 0.00 | 0.62 |
| 15 | 1.90 | 0.77 | 1.03 | 0.45 | 0.00 | 0.00 | 0.67 |
| 16 | 4.69 | 0.34 | 0.13 | 0.12 | 0.00 | 0.00 | 0.00 |
| 17 | 4.81 | 0.79 | 1.02 | 0.25 | 0.02 | 0.00 | 0.39 |
| 18 | 2.14 | 2.81 | 2.87 | 1.59 | 0.00 | 0.00 | 0.00 |
| 19 | 4.73 | 1.18 | 1.63 | 0.36 | 0.04 | 0.55 | 0.59 |
| 20 | 6.00 | 0.92 | 0.85 | 0.20 | 0.02 | 0.74 | 0.41 |
| Average coefficient | 1.00 | 0.99 | 0.41 | 0.01 | 0.10 | 0.26 | |
| Separation | Fraction | Grain Size (mm) | Mass (g) | Picture |
|---|---|---|---|---|
| The sample after crumbling and separating with sieves | >1 | 70.4 |  | |
| Neodymium magnet | Magnetic (I) | >1 | 27.2 |  |
| Non-magnetic (II) | >1 | 43.2 |  | |
| The sample after crumbling and separating with sieves | < 1 | 263.2 |  | |
| Plate separator | Magnetic (III) | < 1 | 2.5 |  |
| Disc separator | Magnetic (IV) | < 1 | 19.8 |  |
| Disc separator | Magnetic (V) | <0.5 | 22.6 |  |
| Non-magnetic (VI) | <0.5 | 207.7 |  | |
| Chemical Element | Chemical Element Content in Fraction (g) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | I | II | III | IV | V | VI | |
| Cu | 79.462 | 2.258 | 32.358 | 7.76 × 10−2 | 3.01 | 4.048 | 37.71 |
| Fe | 27.247 | 23.344 | 0.764 | 1.425 | 1.416 | 0.135 | 0.164 |
| Al | 1.805 | 1.83 × 10−2 | 6.81 × 10−2 | 1 × 10−6 | 0.23 | 0.256 | 1.233 |
| Sn | 1.62 | 0.113 | 0.631 | 3.23 × 10−3 | 3.22 × 10−2 | 1.18 × 10−3 | 0.839 |
| Zn | 3.123 | 0.169 | 2.268 | 7.4×10−2 | 0.134 | 8.42 × 10−2 | 0.394 |
| Nd | 0.508 | 1.58 × 10−3 | 7.9 × 10−5 | 0.338 | 0.159 | 5.22 × 10−3 | 3.99 × 10−3 |
| Au | 0.197 | 2.32 × 10−3 | 6.47 × 10−3 | 8.76 × 10−4 | 1.53 × 10−2 | 7.19 × 10−3 | 0.164 |
| Co | 0.205 | 0.145 | 3.32 × 10−2 | 1.69 × 10−2 | 4.71 × 10−3 | 1.29 × 10−3 | 3.8 × 10−3 |
| Ag | 1.2 × 10−2 | 2.6 × 10−5 | 5.6 × 10−5 | 1.8 × 10−4 | 1.51 × 10−3 | 4.7 × 10−4 | 9.47 × 10−3 |
| Mo | 1.9 × 10−2 | 3.89 × 10−3 | 1.16 × 10−2 | 4.6 × 10−4 | 3.1 × 10−4 | 1.4 × 10−4 | 2.15 × 10−3 |
| Other element (Si, O, etc.) | 219.40 | 1145 | 7059 | 0.563 | 14.79 | 18.06 | 167.17 |
| fraction mass | 333.6 | 27.2 | 43.2 | 2.50 | 19.8 | 22.6 | 207.7 |
| Cu, α = 24.6% | |||||||
| Fraction | γ, % | ∑γ,% | λ, % | β = ∑(γ × λ)/∑γ, % | K = β/α | ε = γ × λ/α, % | ∑ ε = K × ∑γ, % |
| I | 8.42 | 8.42 | 8.30 | 8.30 | 0.337 | 2.84 | 2.84 |
| II | 13.4 | 21.8 | 74.9 | 49.17 | 2.00 | 40.7 | 43.6 |
| III | 0.77 | 22.6 | 3.10 | 47.59 | 1.93 | 0.098 | 43.7 |
| IV | 6.13 | 28.7 | 15.2 | 40.67 | 1.65 | 3.79 | 47.4 |
| V | 7.00 | 35.7 | 17.9 | 36.21 | 1.47 | 5.09 | 52.5 |
| VI | 64.3 | 100 | 18.2 | 24.60 | 1.00 | 47.5 | 100 |
| Fe, α = 8.44% | |||||||
| Fraction | γ,% | ∑γ,% | λ, % | β = ∑(γ × λ)/∑γ, % | K = β/α | ε = γ × λ/α,% | ∑ ε = K × ∑γ, % |
| I | 8.42 | 8.42 | 85.8 | 85.8 | 10.2 | 85.7 | 85.7 |
| II | 13.4 | 21.8 | 1.77 | 34.2 | 4.06 | 2.80 | 88.5 |
| III | 0.77 | 22.6 | 57.0 | 35.0 | 4.15 | 5.23 | 93.7 |
| IV | 6.13 | 28.7 | 7.15 | 29.1 | 3.45 | 5.20 | 98.9 |
| V | 7.00 | 35.7 | 0.597 | 23.5 | 2.78 | 0.495 | 99.4 |
| VI | 64.3 | 100 | 0.079 | 8.44 | 1.00 | 0.602 | 100 |
| Nd, α = 0.16% | |||||||
| Fraction | γ,% | ∑γ,% | λ, % | β = ∑(γ × λ)/∑γ,% | K = β/α | ε = γ × λ/α,% | ∑ ε = K × ∑γ, % |
| I | 8.42 | 8.42 | 0.006 | 0.006 | 0.037 | 0.311 | 0.311 |
| II | 13.4 | 21.8 | 0.0002 | 0.002 | 0.015 | 0.016 | 0.327 |
| III | 0.77 | 22.6 | 13.5 | 0.466 | 2.96 | 66.6 | 66.9 |
| IV | 6.13 | 28.7 | 0.803 | 0.538 | 3.42 | 31.3 | 98.2 |
| V | 7.00 | 35.7 | 0.023 | 0.437 | 2.78 | 1.03 | 99.2 |
| VI | 64.3 | 100 | 0.002 | 0.157 | 1.00 | 0.786 | 100 |
| Co, α = 0.06% | |||||||
| Fraction | γ,% | ∑γ,% | λ, % | β = ∑(γ × λ)/∑γ, % | K = β/α | ε = γ × λ/α,% | ∑ ε = K × ∑γ, % |
| I | 8.42 | 8.42 | 0.533 | 0.533 | 8.40 | 70.8 | 70.8 |
| II | 13.4 | 21.8 | 0.077 | 0.253 | 3.99 | 16.2 | 87.0 |
| III | 0.77 | 22.6 | 0.676 | 0.268 | 4.22 | 8.25 | 95.2 |
| IV | 6.13 | 28.7 | 0.024 | 0.216 | 3.40 | 2.30 | 97.5 |
| V | 7.00 | 35.7 | 0.006 | 0.174 | 2.75 | 0.630 | 98.1 |
| VI | 64.3 | 100 | 0.002 | 0.063 | 1.00 | 1.85 | 100 |
| Mo, α = 0.006% | |||||||
| Fraction | γ,% | ∑γ,% | λ, % | β = ∑(γ × λ)/∑γ, % | K = β/α | ε = γ × λ/α,% | ∑ ε = K × ∑γ, % |
| I | 8.42 | 8.42 | 0.014 | 0.014 | 2.49 | 21.0 | 21.0 |
| II | 13.4 | 21.8 | 0.027 | 0.022 | 3.83 | 62.5 | 83.5 |
| III | 0.77 | 22.6 | 0.018 | 0.022 | 3.81 | 2.48 | 86.0 |
| IV | 6.13 | 28.7 | 0.002 | 0.018 | 3.05 | 1.67 | 87.7 |
| V | 7.00 | 35.7 | 0.001 | 0.014 | 2.48 | 0.755 | 88.4 |
| VI | 64.3 | 100 | 0.001 | 0.006 | 1.00 | 11.6 | 100 |
| Al, α = 0.56% | |||||||
| Fraction | γ, % | ∑γ, % | λ, % | β = ∑(γ × λ)/∑γ, % | K = β/α | ε = γ × λ/α, % | ∑ ε = K × ∑γ, % |
| I | 8.42 | 8.42 | 0.067 | 0.067 | 0.120 | 1.0 | 1.0 |
| II | 13.4 | 21.8 | 0.158 | 0.123 | 0.220 | 3.8 | 4.8 |
| III | 0.77 | 22.6 | 0.000 | 0.119 | 0.212 | 0.000 | 4.8 |
| IV | 6.13 | 28.7 | 1.16 | 0.341 | 0.611 | 12.7 | 17.5 |
| V | 7.00 | 35.7 | 1.13 | 0.496 | 0.888 | 14.2 | 31.7 |
| VI | 64.3 | 100 | 0.594 | 0.559 | 1.00 | 68.3 | 100 |
| Zn, α = 0.97% | |||||||
| Fraction | γ, % | ∑γ, % | λ, % | β = ∑ γ × λ/∑ γ, % | K = β/α | ε = γ × λ/α, % | ∑ ε = K × ∑γ, % |
| I | 8.42 | 8.42 | 0.621 | 0.621 | 0.643 | 5.41 | 5.41 |
| II | 13.4 | 21.8 | 5.25 | 3,46 | 3.58 | 72.6 | 78,0 |
| III | 0.77 | 22.6 | 2.96 | 3.44 | 3.56 | 2.37 | 80.4 |
| IV | 6.13 | 28.7 | 0.677 | 2.85 | 2.95 | 4.29 | 84.7 |
| V | 7.00 | 35.7 | 0.373 | 2.37 | 2.45 | 2.70 | 87.4 |
| VI | 64.3 | 100 | 0.190 | 0.967 | 1.00 | 12.6 | 100 |
| Sn, α = 0.50% | |||||||
| Fraction | γ, % | ∑γ, % | λ, % | β = ∑ γ × λ/∑ γ, % | K = β/α | ε = γ × λ/α, % | ∑ ε = K × ∑γ, % |
| I | 8.42 | 8.42 | 0.415 | 0.415 | 0.829 | 6.98 | 6.98 |
| II | 13.4 | 21.8 | 1.46 | 1.06 | 2.11 | 39.0 | 45.9 |
| III | 0.77 | 22.6 | 0.129 | 1.03 | 2.04 | 0.199 | 46.1 |
| IV | 6.13 | 28.7 | 0.163 | 0.841 | 1.68 | 1.99 | 48.1 |
| V | 7.00 | 35.7 | 0.005 | 0.677 | 1.35 | 0.073 | 48.2 |
| VI | 64.3 | 100 | 0.404 | 0.501 | 1.00 | 51.8 | 100 |
| Au, α = 0.06% | |||||||
| Fraction | γ, % | ∑γ, % | λ, % | β = ∑ γ × λ/∑ γ, % | K = β/α | ε = γ × λ/α, % | ∑ ε = K × ∑γ, % |
| I | 8.42 | 8.42 | 0.009 | 0.009 | 0.140 | 1.18 | 1.18 |
| II | 13.4 | 21.8 | 0.015 | 0.012 | 0.206 | 3.30 | 4.48 |
| III | 0.77 | 22.6 | 0.035 | 0.013 | 0.218 | 0.447 | 4.93 |
| IV | 6.13 | 28.7 | 0.077 | 0.027 | 0.443 | 7.80 | 12.7 |
| V | 7.00 | 35.7 | 0.032 | 0.028 | 0.459 | 3.67 | 16.4 |
| VI | 64.3 | 100 | 0.079 | 0.061 | 1.00 | 83.6 | 100 |
| Ag, α = 0.004% | |||||||
| Fraction | γ, % | ∑γ, % | λ, % | β = ∑ γ × λ/∑ γ, % | K = β/α | ε = γ × λ/α, % | ∑ ε = K × ∑γ, % |
| I | 8.42 | 8.42 | 0.0001 | 0.0001 | 0.026 | 0.222 | 0.222 |
| II | 13.4 | 21.8 | 0.0001 | 0.0001 | 0.032 | 0.478 | 0.700 |
| III | 0.77 | 22.6 | 0.007 | 0.0004 | 0.099 | 1.54 | 2.24 |
| IV | 6.13 | 28.7 | 0.008 | 0.0019 | 0.527 | 12.9 | 15.1 |
| V | 7.00 | 35.7 | 0.002 | 0.0019 | 0.536 | 4.01 | 19.1 |
| VI | 64.3 | 100 | 0.005 | 0.0036 | 1.00 | 80.9 | 100 |
| Fraction | Crystalline Phase Identified | ||||
|---|---|---|---|---|---|
| Si | Cu | Fe | Sn | Others | |
| I | - | Cu—42.2% | Fe—57.3% | - | W—0.5% |
| II | Si—1.1% | Cu—46.5% Cu (Zn)—23.9% Cu (Pb, Sn, Zn)—11.3% | - | - | Al—13.6% C—3.6% |
| III | - | - | Fe14Nd2B—31.4% Fe3O4—25.7% | SnO2—5.7% | Ni—37.2% |
| IV | SiO2—62.3% | Cu2O—8.9% CuO—4.8% Cu—5.6% | CuFe—3.0% | SnO3Sr—2.7% SnO2—2.2% | BaTiO3—10.6% |
| V | SiO2—61.0% | Cu2O—25.3% Tl0.01Cu3.99 —6.7% CuO—3.6% CuGaTe2—1.4% | - | SnO2—2.2% | - |
| VI | SiO2—59.8% | Cu2O—29.4% Tl0.01Cu3.99 —5.1% CuO—2.1% | - | SnO2—3.5% | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żukowski, W.; Kowalska, A.; Wrona, J. High-Temperature Fluidized Bed Processing of Waste Electrical and Electronic Equipment (WEEE) as a Way to Recover Raw Materials. Energies 2021, 14, 5639. https://doi.org/10.3390/en14185639
Żukowski W, Kowalska A, Wrona J. High-Temperature Fluidized Bed Processing of Waste Electrical and Electronic Equipment (WEEE) as a Way to Recover Raw Materials. Energies. 2021; 14(18):5639. https://doi.org/10.3390/en14185639
Chicago/Turabian StyleŻukowski, Witold, Amelia Kowalska, and Jan Wrona. 2021. "High-Temperature Fluidized Bed Processing of Waste Electrical and Electronic Equipment (WEEE) as a Way to Recover Raw Materials" Energies 14, no. 18: 5639. https://doi.org/10.3390/en14185639