The Effect of Ammonia Toxicity on Methane Production of a Full-Scale Biogas Plant—An Estimation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates and Inoculum
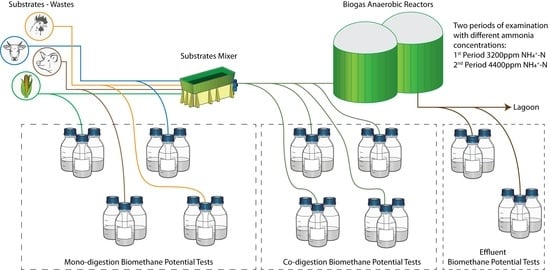
2.2. Experimental Design
2.3. BMP Tests
2.4. Analytical Methods
3. Results and Discussion
3.1. BMPs of Substrates and Comparison with the Actual Methane Production of the Biogas Plant—Preliminary Assessment of Ammonia Toxicity
3.2. Comparison of Methane Production by Anaerobic Co-Digestion of Substrates with Different Ammonia Concentrations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnau, Á.S.; Pflüger, S.; Giacomazzi, M.; Decorte, M.; Genua, M. Annual Report 2020. European Biogas Association: Brussels, Belgium. Available online: https://www.europeanbiogas.eu/eba-annual-report-2020/ (accessed on 5 July 2021).
- Xue, S.; Song, J.; Wang, X.; Shang, Z.; Sheng, C.; Li, C.; Zhu, Y.; Liu, J. A systematic comparison of biogas development and related policies between China and Europe and corresponding insights. Renew. Sustain. Energy Rev. 2020, 117, 109474. [Google Scholar] [CrossRef]
- Kougias, P.; Kotsopoulos, T.; Martzopoulos, G. Anaerobic co-digestion of pig waste with olive mill wastewater under various mixing conditions. Fresenius Environ. Bull. 2010, 19, 1682–1686. [Google Scholar]
- Wellisch, M.; Green, J.; McCabe, B.; Rasi, S.; Siemens, W.; Ammenberg, J.; Liebetrau, J.; Bochmann, G.; Murphy, J.D. Drivers for Successful and Sustainable Biogas Projects: International Perspectives. In Proceedings of the Report of a Symposium in Association with Canadian Biogas Association held on line via GoToMeetings, 26 March 2020. [Google Scholar]
- Dagnall, S.; Hill, J.; Pegg, D. Resource mapping and analysis of farm livestock manures—Assessing the opportunities for biomass-to-energy schemes. Bioresour. Technol. 2000, 71, 225–234. [Google Scholar] [CrossRef]
- Bücker, F.; Marder, M.; Peiter, M.R.; Lehn, D.N.; Esquerdo, V.M.; Antonio de Almeida Pinto, L.; Konrad, O. Fish waste: An efficient alternative to biogas and methane production in an anaerobic mono-digestion system. Renew. Energy 2020, 147, 798–805. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, W.; He, Y.; Zhang, R.; Liu, G. Effect of ammonia on methane production, methanogenesis pathway, microbial community and reactor performance under mesophilic and thermophilic conditions. Renew. Energy 2018, 125, 915–925. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ahring, B.K. Thermophilic anaerobic digestion of livestock waste: The effect of ammonia. Appl. Microbiol. Biotechnol. 1993, 38, 560–564. [Google Scholar] [CrossRef]
- Fotidis, I.A.; Karakashev, D.; Angelidaki, I. The dominant acetate degradation pathway/methanogenic composition in full-scale anaerobic digesters operating under different ammonia levels. Int. J. Environ. Sci. Technol. 2014, 11, 2087–2094. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, H.B.; Angelidaki, I. Codigestion of manure and industrial organic waste at centralized biogas plants: Process imbalances and limitations. Water Sci. Technol. 2008, 58, 1521–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braz, G.H.R.; Fernandez-Gonzalez, N.; Lema, J.M.; Carballa, M. Organic overloading affects the microbial interactions during anaerobic digestion in sewage sludge reactors. Chemosphere 2019, 222, 323–332. [Google Scholar] [CrossRef]
- Polizzi, C.; Alatriste-Mondragón, F.; Munz, G. The role of organic load and ammonia inhibition in anaerobic digestion of tannery fleshing. Water Resour. Ind. 2018, 19, 25–34. [Google Scholar] [CrossRef]
- Akbulut, A. Techno-economic analysis of electricity and heat generation from farm-scale biogas plant: Çiçekdağı case study. Energy 2012, 44, 381–390. [Google Scholar] [CrossRef]
- Urbanowska, A.; Kabsch-Korbutowicz, M. The Use of Flat Ceramic Membranes for Purification of the Liquid Fraction of the Digestate from Municipal Waste Biogas Plants. Energies 2021, 14, 3947. [Google Scholar] [CrossRef]
- Urbanowska, A.; Kabsch-Korbutowicz, M.; Wnukowski, M.; Seruga, P.; Baranowski, M.; Pawlak-Kruczek, H.; Serafin-Tkaczuk, M.; Krochmalny, K.; Niedzwiecki, L. Treatment of Liquid By-Products of Hydrothermal Carbonization (HTC) of Agricultural Digestate Using Membrane Separation. Energies 2020, 13, 262. [Google Scholar] [CrossRef] [Green Version]
- Urbanowska, A.; Polowczyk, I.; Kabsch-Korbutowicz, M.; Seruga, P. Characteristics of Changes in Particle Size and Zeta Potential of the Digestate Fraction from the Municipal Waste Biogas Plant Treated with the Use of Chemical Coagulation/Precipitation Processes. Energies 2020, 13, 5861. [Google Scholar] [CrossRef]
- Koch, K.; Hafner, S.D.; Weinrich, S.; Astals, S.; Holliger, C. Power and Limitations of Biochemical Methane Potential (BMP) Tests. Front. Energy Res. 2020, 8, 63. [Google Scholar] [CrossRef]
- Mönch-Tegeder, M.; Lemmer, A.; Oechsner, H. Enhancement of methane production with horse manure supplement and pretreatment in a full-scale biogas process. Energy 2014, 73, 523–530. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Kalamaras, S.D.; Kotsopoulos, T.A. Anaerobic co-digestion of cattle manure and alternative crops for the substitution of maize in South Europe. Bioresour. Technol. 2014, 172, 68–75. [Google Scholar] [CrossRef]
- Liu, J.; van Gorp, R.; Nistor, M. The New Gas Endeavour System from Bioprocess Control AB for In Vitro Assessment of Animal feeds. In Proceedings of the 9th Nordic Feed Science Conference, Uppsala, Sweden, 12–13 June 2018; Udén, P., Eriksson, T., Spörndly, R., Rustas, B.-O., Liljeholm, M., Eds.; Swedish University of Agricultural Sciences, Department of Animal Nutrition and Management: Uppsala, Sweden, 2018; pp. 135–142. [Google Scholar]
- Holliger, C.; Fruteau de Laclos, H.; Hack, G. Methane Production of Full-Scale Anaerobic Digestion Plants Calculated from Substrate’s Biomethane Potentials Compares Well with the One Measured On-Site. Front. Energy Res. 2017, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Adghim, M.; Abdallah, M.; Saad, S.; Shanableh, A.; Sartaj, M. Assessment of the biochemical methane potential of mono- and co-digested dairy farm wastes. Waste Manag. Res. 2020, 38, 88–99. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, J.M.; Salazar, L.; Castro, L.; Escalante, H. Anaerobic co-digestion of vinasse and chicken manure: Alternative for Colombian agrowaste management. Rev. Colomb. Biotecnol. 2016, 18, 6–12. [Google Scholar]
- Siles, J.A.; Martín, M.A.; Chica, A.F.; Martín, A. Anaerobic co-digestion of glycerol and wastewater derived from biodiesel manufacturing. Bioresour. Technol. 2010, 101, 6315–6321. [Google Scholar] [CrossRef]
- Kreuger, E.; Nges, I.A.; Björnsson, L. Ensiling of crops for biogas production: Effects on methane yield and total solids determination. Biotechnol. Biofuels 2011, 4, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escalante, H.; Castro, L.; Amaya, M.P.; Jaimes, L.; Jaimes-Estévez, J. Anaerobic digestion of cheese whey: Energetic and nutritional potential for the dairy sector in developing countries. Waste Manag. 2018, 71, 711–718. [Google Scholar] [CrossRef]
- El Achkar, J.H.; Lendormi, T.; Hobaika, Z.; Salameh, D.; Louka, N.; Maroun, R.G.; Lanoisellé, J.-L. Anaerobic digestion of grape pomace: Biochemical characterization of the fractions and methane production in batch and continuous digesters. Waste Manag. 2016, 50, 275–282. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Walter, A.; Ebner, C.; Insam, H. Investigation into the effect of high concentrations of volatile fatty acids in anaerobic digestion on methanogenic communities. Waste Manag. 2014, 34, 2080–2089. [Google Scholar] [CrossRef] [Green Version]
- Ahring, B.K.; Sandberg, M.; Angelidaki, I. Volatile fatty acids as indicators of process imbalance in anaerobic digestors. Appl. Microbiol. Biotechnol. 1995, 43, 559–565. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Demirel, B.; Yenigün, O. The Effects of Change in Volatile Fatty Acid (VFA) Composition on Methanogenic Upflow Filter Reactor (UFAF) Performance. Environ. Technol. 2002, 23, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Karakashev, D.; Batstone, D.J.; Plugge, C.M.; Stams, A.J.M. Chapter Sixteen—Biomethanation and Its Potential. In Methods in Enzymology; Rosenzweig, A.C., Ragsdale, S.W., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 494, pp. 327–351. [Google Scholar]
- Astals, S.; Batstone, D.J.; Mata-Alvarez, J.; Jensen, P.D. Identification of synergistic impacts during anaerobic co-digestion of organic wastes. Bioresour. Technol. 2014, 169, 421–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.; Cheng, Q.-S.; Sun, Z.-Y.; Tang, Y.-Q.; Kida, K. Effects of ammonium and/or sulfide on methane production from acetate or propionate using biochemical methane potential tests. J. Biosci. Bioeng. 2019, 127, 345–352. [Google Scholar] [CrossRef] [PubMed]



| Substrate | TS (%w/w) | VS (% TS) | NH4+–N (mg·L−1) | TKN (mg·L−1) | pH | Period Utilized |
|---|---|---|---|---|---|---|
| Cattle manure | 5.78 ± 0.06 | 79.67 ± 0.58 | 486 ± 14 | 2112.63 ± 84 | 6.65 | 1st and 2nd |
| Poultry manure | 24.55 ± 0.25 | 72.33 ± 0.58 | 4533 ± 156 | 19,074.97+125 | 7.5 | 1st and 2nd |
| Pig manure | 4.45 ± 0.07 | 73.48 ± 0.28 | 1791 ± 45 | 3262 ± 24 | 6.78 | 1st and 2nd |
| Whey | 6.72 ± 0.06 | 84.05 ± 1 | 38 ± 4 | 1277.77 ± 45 | 4.72 | 1st and 2nd |
| Fruit pulp | 14.29 ± 0.41 | 97.34 ± 0.57 | 224 ± 42 | 1568 ± 14 | 3.5 | 1st |
| Corn silage | 36.55 ± 0.24 | 96.5 ± 0.71 | 69 ± 8 | 4300 ± 131 | 3.83 | 1st and 2nd |
| Spent grapes | 44.75 ± 0.78 | 71.00 ± 9.19 | 258 ± 8 | 3072 ± 158 | 3.94 | 1st |
| Glycerine | 85.34 ± 6.71 | 81.38 ± 6.55 | NM * | 1149 ± 394 | 8.6 | 2nd |
| Dry poultry manure | 74.88 ± 15.04 | 63.81 ± 10.76 | NM * | 31,217 ± 16,160 | 8.2 | 2nd |
| Biodiesel soap residue | 10.27 ± 4.41 | 10.11 ± 4.36 | 12 ± 1.04 | 229 ± 185 | 9.3 | 2nd |
| Dried digestate | 25.95 ± 2.01 | 23.71 ± 2.76 | 5584 ± 1455 | 23,300 ± 3412 | 8.7 | 2nd |
| Dough waste | 74.51 | 73.52 | NM | 18,432 | 4.3 | 2nd |
| Inoculum | 4.94 ± 0.05 | 71.67 ± 0.58 | 3200 ± 112 | 5176.5 ± 78 | 8.14 |
| Substrate | 1st Period (30 days) | 2nd Period (30 days) * | ||||
|---|---|---|---|---|---|---|
| Input (t) | m3 CH4 t−1 | Total CH4 (m3) | Input (t) | m3 CH4 t−1 | Total CH4 (m3) | |
| Cattle manure | 5313.60 | 15.68 | 83,317.47 | 3747.25 | 16.98 | 63,628.31 |
| Poultry manure | 943.20 | 62.01 | 58,490.82 | 990.69 | 79.30 | 78,561.72 |
| Pig manure | 196.80 | 12.84 | 2526.33 | 222.81 | 6.33 | 1411.12 |
| Whey | 578.40 | 32.31 | 18,687.19 | 54.66 | 19.83 | 1084.02 |
| Fruit pulp | 158.40 | 51.84 | 8210.67 | nu ** | nu | nu |
| Corn silage | 150.00 | 128.10 | 19,215.18 | 212.30 | 117.48 | 24,941.00 |
| Spent grapes | 60.00 | 8.51 | 510.47 | nu | nu | nu |
| Glycerine | nu | nu | nu | 19.82 | 428.83 | 8999.41 |
| Dry poultry manure | nu | nu | nu | 222.00 | 93.44 | 20,743.68 |
| Biodiesel soap residue | nu | nu | nu | 59.82 | 16.77 | 1003.14 |
| Dry digestate | nu | nu | nu | 170.53 | 43.13 | 7354.96 |
| Dough waste | nu | nu | nu | 32.62 | 268.58 | 8760.99 |
| Total CH4 production | 190,958.13 | Total CH4 production | 215,988.35 | |||
| Period | NH4+–N Concentration (mg·L−1) | Actual Biogas Plant’s CH4 Production (m3) | Total Calculated CH4 Production (m3) * | Reduction Rate (%) |
|---|---|---|---|---|
| First Period of study | 3200 | 172,136.61 | 190,958.13 | 9.9 |
| Second period of study | 4400 | 172,136.61 | 215,988.35 | 20.3 |
| Volatile Fatty Acid-VFA | Effluent 1st Period | Effluent 2nd Period |
|---|---|---|
| Acetic acid (mM) | 3.74 | 41.86 |
| Propionic acid (mM) | 0.08 | 22.45 |
| Isobutyric acid (mM) | ND | 0.38 |
| Butyric acid (mM) | ND | 0.11 |
| Isovaleric acid (mM) | 0.02 | 0.72 |
| n-Valeric acid (mM) | ND | 0.09 |
| Isocaproic acid (mM) | 0.06 | 0.03 |
| n-Caproic acid (mM) | ND | 0.03 |
| Heptanoic acid (mM) | 0.01 | 0.02 |
| Treatment (Initial and Final NH4+−N mg·L−1) | 3N (3358–4580) | 4N (4331–5222) | 5N (5304–6437) |
|---|---|---|---|
| 2N (2060–3157) | −14.02% CH4 | −20.56% CH4 | −35.00% CH4 |
| 3N (3358–4580) | −7.60% CH4 | −24.40% CH4 | |
| 4N (4331–5222) | −18.17% CH4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalamaras, S.D.; Vitoulis, G.; Christou, M.L.; Sfetsas, T.; Tziakas, S.; Fragos, V.; Samaras, P.; Kotsopoulos, T.A. The Effect of Ammonia Toxicity on Methane Production of a Full-Scale Biogas Plant—An Estimation Method. Energies 2021, 14, 5031. https://doi.org/10.3390/en14165031
Kalamaras SD, Vitoulis G, Christou ML, Sfetsas T, Tziakas S, Fragos V, Samaras P, Kotsopoulos TA. The Effect of Ammonia Toxicity on Methane Production of a Full-Scale Biogas Plant—An Estimation Method. Energies. 2021; 14(16):5031. https://doi.org/10.3390/en14165031
Chicago/Turabian StyleKalamaras, Sotirios D., Georgios Vitoulis, Maria Lida Christou, Themistoklis Sfetsas, Spiridon Tziakas, Vassilios Fragos, Petros Samaras, and Thomas A. Kotsopoulos. 2021. "The Effect of Ammonia Toxicity on Methane Production of a Full-Scale Biogas Plant—An Estimation Method" Energies 14, no. 16: 5031. https://doi.org/10.3390/en14165031