Biogas Production from Coffee Pulp and Chicken Feathers Using Liquid- and Solid-State Anaerobic Digestions
Abstract
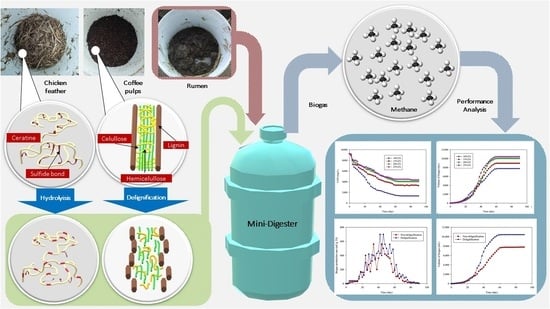
:1. Introduction
2. Materials and Methods
2.1. Materials and Tools
2.2. Raw Material Preparation
2.3. Nitrogen Content
2.4. Carbon Content
2.5. Biogas Production Process
2.6. COD Analysis
2.7. Kinetics of Biogas Production Using The Gompertz Model
- y(t): The cumulative biogas yield at a digestion time t days (mL/g·TS)
- ym: The biogas production potential (mL/g·TS·day)
- U: The maximum biogas production rate (mL/g·TS·day)
- λ: Lag phase period or minimum time to produce biogas (days)
- t: Cumulative time for biogas production (days)
- e: Mathematical constant (2.718282)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Different TS Percentages on COD Reduction and Biogas Production
3.1.1. COD Reduction
3.1.2. Biogas Production
3.2. Effect of Different C/N Ratios on COD Reduction and Biogas Production
3.2.1. COD Reduction
3.2.2. Biogas Production
3.3. Effect of Pretreatment or Delignification on COD Reduction and Biogas Production
3.3.1. COD Reduction
3.3.2. Biogas Production
3.4. Kinetics of Biogas Production Using The Gompertz Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Looney, B. BP Statistical Review of World Energy. BP Statistical Review, 70th ed.; London, UK, 2021; Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2021-full-report.pdf (accessed on 28 June 2021).
- Adib, R. Renewables 2020 Global Status Report; Athens, Greece, 2020. Available online: https://www.ren21.net/wp-content/uploads/2019/05/gsr_2020_full_report_en.pdf (accessed on 28 June 2021).
- Hoogwijk, M.; Graus, W. Global Potential of Renewable Energy Sources: A Literature Assessment; Ecofys: Utrecht, The Netherlands, 2008. [Google Scholar]
- Sumardiono, S.; Budi Riyanta, A.; Hawali Abdul Matin, H.; Djoko Kusworo, T.; Jos, B. Budiyono Increasing biogas production from sugar cane baggase by anaerobic co-digestion with animal manure. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2017; Volume 101, p. 02014. [Google Scholar]
- Mézes, L.; Tamás, J.; Juhász, C.; Mátyás, B. Feather waste recycle for Biogas production. In Proceedings of the 2nd International Conference on Sustainable Solid Waste Management, Athen, Greece, 12–14 June 2014. [Google Scholar]
- Arifan, F.; Abdullah, A.; Sumardiono, S. Effect of Organic Waste Addition into Animal Manure on Biogas Production Using Anaerobic Digestion Method. Int. J. Renew. Eng. Dev. 2021, 10, 623–633. [Google Scholar] [CrossRef]
- Subramaniyam, R.; Vimala, R. Solid state and submerged fermentation for the production of bioactive substances: A comparative study. Int. J. Sci. Nat. 2012, 3, 480–486. [Google Scholar]
- Didanna, H.L. A critical review on feed value of coffee waste for livestock feeding. World J. Biol. Biol. Sci. 2014, 2, 72–86. [Google Scholar]
- Cheong, C.W.; Lee, Y.S.; Ahmad, S.A.; Ooi, P.T.; Phang, L.Y. Chicken feather valorization by thermal alkaline pretreatment followed by enzymatic hydrolysis for protein-rich hydrolysate production. Waste Manag. 2018, 79, 658–666. [Google Scholar] [CrossRef]
- Manan, M.A.; Webb, C. Design aspects of solid state fermentation as applied to microbial bioprocessing. J. Appl. Biotechnol. Bioeng. 2017, 4, 91. [Google Scholar]
- Dahunsi, S.O. Mechanical pretreatment of lignocelluloses for enhanced biogas production: Methane yield prediction from biomass structural components. Bioresour. Technol. 2019, 280, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Kangle, K.M.; Kore, S.V.; Kore, V.S.; Kulkarni, G.S. Recent trends in anaerobic codigestion: A review. J. Environ. Res. Technol. 2012, 2, 210–219. [Google Scholar]
- Abunde Neba, F.; Asiedu, N.Y.; Addo, A.; Morken, J.; Østerhus, S.W.; Seidu, R. Simulation of two-dimensional attainable regions and its application to model digester structures for maximum stability of anaerobic treatment process. Water Res. 2019, 163, 114891. [Google Scholar] [CrossRef]
- Mulat, D.G.; Horn, S.J. Biogas production from lignin via anaerobic digestion. In Lignin Valorization: Emerging Approaches, 1st ed.; Royal Society of Chemistry: Chicago, IL, USA, 2018; pp. 391–412. [Google Scholar]
- Yu, Q.; Liu, R.; Li, K.; Ma, R. A review of crop straw pretreatment methods for biogas production by anaerobic digestion in China. Renew. Sustain. Eng. Rev. 2019, 107, 51–58. [Google Scholar] [CrossRef]
- Jos, B.; Hundagi, F.; Wisudawati, R.P.; Budiyono; Sumardiono, S. Study of C/N Ratio Effect on Biogas Production of Carica Solid Waste by SS-AD Method and LS-AD. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 156, p. 03055. [Google Scholar] [CrossRef] [Green Version]
- Sumardiono, S.; Pudjihastuti, I.; Budiyono; Hartanto, H.; Sophiana, I.C. Combination process method of lactic acid hydrolysis and hydrogen peroxide oxidation for cassava starch modification. AIP Conf. Proc. 2017, 1840, 060006. [Google Scholar] [CrossRef]
- Ilaboya, I.R.; Asekhame, F.F.; Ezugwu, M.O.; Erameh, A.A.; Omofuma, F.E. Studies on biogas generation from agricultural waste; analysis of the effects of alkaline on gas generation. World Appl. Sci. J. 2010, 9, 537–545. [Google Scholar]
- Sumardiono, S.; Pudjihastuti, I.; Jos, B.; Taufani, M.; Yahya, F. Modification of cassava starch using combination process lactic acid hydrolysis and microwave heating to increase coated peanut expansion quality. AIP Conf. Proc. 2017, 1840, 060005. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; APHA-AWWA-WEF: Washington, DC, USA, 2017. [Google Scholar]
- Sumardiono, S.; Pudjihastuti, I.; Poerwoprajitno, A.R.; Suswadi, M.S. Physicochemical properties of analog rice from composite flour: Cassava, green bean and hanjeli. World Appl. Sci. J. 2014, 32, 1140–1146. [Google Scholar]
- Walkley, A.; Black, I.A. An Examination of The Degtjareff Method for Determining Soil Organic Matter, and A Proposed Modification of The Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Sumardiono, S.; Adisukmo, G.; Hanif, M.; Budiyono, B.; Cahyono, H. Effects of Pretreatment and Ratio of Solid Sago Waste to Rumen on Biogas Production through Solid-State Anaerobic Digestion. Sustainability 2021, 13, 7491. [Google Scholar] [CrossRef]
- Saelor, S.; Kongjan, P.; O-Thong, S. Biogas Production from Anaerobic Co-digestion of Palm Oil Mill Effluent and Empty Fruit Bunches. Eng. Procedia 2017, 138, 717–722. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A review of the processes, parameters, and optimization of anaerobic digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jos, B.; Farhan, H.; Ayu, N.D.; Budiyono; Sumardiono, S. Biogas Production from Palm Oil Fruit Bunch in Anaerobic Biodigester through Liquid State (LS-AD) and Solid State (SS-AD) Method. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 156, p. 03043. [Google Scholar]
- Prasetyo, T.; Sumardiono, S.; Aji, H.A.; Pratama, A.Y. Effect of C/N Ratio and pH on biogas production from industrial cassava starch wastewater through anaerobic process. Adv. Sci. Lett. 2017, 23, 5810–5814. [Google Scholar] [CrossRef]
- Mel, M.; Yong, A.S.H.; Avicenna; Ihsan, S.I.; Setyobudi, R.H. Simulation Study for Economic Analysis of Biogas Production from Agricultural Biomass. Energy Procedia 2015, 65, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [Green Version]
- Saragih, F.N.A.; Priadi, C.R.; Adityosulindro, S.; Abdillah, A.; Islami, B.B. The effectiveness of anaerobic digestion process by thermal pre-treatment on food waste as a substrate. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2019; Volume 251, p. 012014. [Google Scholar]
- Budiyono, B.; Manthia, F.; Amalin, N.; Matin, H.H.A.; Sumardiono, S. Production of Biogas from Organic Fruit Waste in Anaerobic Digester using Ruminant as the Inoculum. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 156, p. 03053. [Google Scholar]
- Rennuit, C.; Triolo, J.M.; Eriksen, S.; Jimenez, J.; Carrère, H.; Hafner, S.D. Comparison of pre- and inter-stage aerobic treatment of wastewater sludge: Effects on biogas production and COD removal. Bioresour. Technol. 2018, 247, 332–339. [Google Scholar] [CrossRef]
- Gao, S.; Hu, C.; Sun, S.; Xu, J.; Zhao, Y.; Zhang, H. Performance of piggery wastewater treatment and biogas upgrading by three microalgal cultivation technologies under different initial COD concentration. Energy 2018, 165, 360–369. [Google Scholar] [CrossRef]
- Ali Shah, F.; Mahmood, Q.; Maroof Shah, M.; Pervez, A.; Ahmad Asad, S. Microbial ecology of anaerobic digesters: The key players of anaerobiosis. Sci. World J. 2014, 2014, 183752. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Li, J.; Nies, L.; Zhang, L. Research advances in dry anaerobic digestion process of solid organic wastes. Afr. J. Biotechnol. 2011, 10, 14242–14253. [Google Scholar]
- Tufaner, F.; Avşar, Y. Effects of co-substrate on biogas production from cattle manure: A review. Int. J. Environ. Sci. Technol. 2016, 13, 2303–2312. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.; Li, Y. Solid state anaerobic co-digestion of yard waste and food waste for biogas production. Bioresour. Technol. 2013, 127, 275–280. [Google Scholar] [CrossRef]
- Bhattacharya, T.K.; Mishra, T.N. Biodegradability of dairy cattle manure under dry anaerobic fermentation process. J. Inst. Eng. Agric. Eng. Div. 2003, 84, 9–10. [Google Scholar]
- Fagbohungbe, M.O.; Onyeri, C.A.; Semple, K.T. Co-fermentation of whey permeates and cattle slurry using a partitioned up-flow anaerobic digestion tank. Energy 2019, 185, 567–572. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, H.; Yang, H.; Wang, H.; Zheng, D.; Liu, Y.; Pu, X.; Deng, L. Improved biogas production of dry anaerobic digestion of swine manure. Bioresour. Technol. 2019, 294, 122188. [Google Scholar] [CrossRef]
- Zeshan; Karthikeyan, O.P.; Visvanathan, C. Effect of C/N ratio and ammonia-N accumulation in a pilot-scale thermophilic dry anaerobic digester. Bioresour. Technol. 2012, 113, 294–302. [Google Scholar] [CrossRef]
- Dioha, I.J.; Ikeme, C.H.; Nafi’u, T.; Soba, N.I.; Yusuf, M.B.S. Effect of carbon to nitrogen ratio on biogas production. Int. Res. J. Nat. Sci. 2013, 1, 1–10. [Google Scholar]
- Wang, X.; Lu, X.; Li, F.; Yang, G. Effects of temperature and Carbon-Nitrogen (C/N) ratio on the performance of anaerobic co-digestion of dairy manure, chicken manure and rice straw: Focusing on ammonia inhibition. PLoS ONE 2014, 9, e97265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Wang, X.; Sun, S.; Zhao, Y.; Hu, C. Effects of influent C/N ratios and treatment technologies on integral biogas upgrading and pollutants removal from synthetic domestic sewage. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kovács, E.; Wirth, R.; Maróti, G.; Bagi, Z.; Nagy, K.; Minárovits, J.; Rákhely, G.; Kovács, K.L. Augmented biogas production from protein-rich substrates and associated metagenomic changes. Bioresour. Technol. 2015, 178, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Sukma, A.; Jos., B.; Sumardiono, S. Kinetic of biomass growth and protein formation on rice bran fermentation using Rhizopus oryzae. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 156, p. 01023. [Google Scholar]
- Sumardiono, S.; Mardiani, D.T. The effect of microwave power and heating time pretreatment on biogas production from fresh and dried water hyacinth (Eichhornia crassipes). AIP Conf. Proc. 2015, 1699, 050018. [Google Scholar]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- Abraham, A.; Mathew, A.K.; Park, H.; Choi, O.; Sindhu, R.; Parameswaran, B.; Pandey, A.; Park, J.H.; Sang, B.I. Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Ajiboye, A.V.; Lasisi, K.H.; Babatola, J.O. Evaluation of the effect of sodium hydroxide solution on biogas yield of anaerobic digestion of poultry waste and the digestate. Int. J. Eng. Water Resour. 2018, 2, 23–31. [Google Scholar] [CrossRef]
- Singh, R.; Karki, A.B.; Nath Shrestha, J. Production of Biogas from Poultry Waste. J. Renew. Energy Smart Grid Technol. 2008, 3, 11–20. [Google Scholar]
- Wardhani, D.H.; Vázquez, J.A.; Ramdani, D.A.; Lutfiati, A.; Aryanti, N.; Cahyono, H. Enzymatic purification of glucomannan from amorphophallus oncophyllus using A-amylase. Biosci. J. 2019, 35, 277–288. [Google Scholar] [CrossRef]
- Ghatak, M.D.; Mahanta, P. Kinetic assessment of biogas production from lignocellulosic biomasses. Int. J. Eng. Adv. Technol. 2014, 3, 244–249. [Google Scholar]
- Vasmara, C.; Cianchetta, S.; Marchetti, R.; Ceotto, E.; Galletti, S. Potassium Hydroxyde Pre-Treatment Enhances Methane Yield from Giant Reed (Arundo donax L.). Energies 2021, 14, 630. [Google Scholar] [CrossRef]
- Budiyono, B.; Primaloka, A.D.; Ardhannari, A.; Matin, H.H.A.; Sumardiono, S. Study of Biogas Production from Cassava Industrial Waste by Anaerobic Process. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 156, p. 03052. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Tang, X.; Zhao, K.; Balan, V.; Zhu, Q. Biogas Production from Anaerobic Co-Digestion of Spent Mushroom Substrate with Different Livestock Manure. Energies 2021, 14, 570. [Google Scholar] [CrossRef]













| No. | %Total Solid (TS) * | Solid | Chemical Pretreatment | Ratio C/N (g/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10% ** | 15% | 20% | 25% | Coffee Pulp | Chicken Feather | Cow Feces | Delignification | Non-Delignification | NaOH *** | 25 | 30 | Urea | |
| 1 | 60 g | 26.66 g | 13.33 g | 20 g | v | v | 21.68 g | ||||||
| 2 | 60 g | 26.66 g | 13.33 g | 20 g | v | v | 7.88 g | ||||||
| 3 | 60 g | 26.66 g | 13.33 g | 20 g | 0.150 N | 3.00 g | v | 21.68 g | |||||
| 4 | 60 g | 26.66 g | 13.33 g | 20 g | 0.150 N | 3.00 g | v | 7.88 g | |||||
| 5 | 90 g | 40.00 g | 20.00 g | 30 g | v | v | 32.52 g | ||||||
| 6 | 90 g | 40.00 g | 20.00 g | 30 g | v | v | 11.82 g | ||||||
| 7 | 90 g | 40.00 g | 20.00 g | 30 g | 0.225 N | 4.50 g | v | 32.52 g | |||||
| 8 | 90 g | 40.00 g | 20.00 g | 30 g | 0.225 N | 4.50 g | v | 11.82 g | |||||
| 9 | 120 g | 53.33 g | 26.66 g | 40 g | v | v | 43.36 g | ||||||
| 10 | 120 g | 53.33 g | 26.66 g | 40 g | v | v | 15.77 g | ||||||
| 11 | 120 g | 53.33 g | 26.66 g | 40 g | 0.300 N | 6.00 g | v | 43.36 g | |||||
| 12 | 120 g | 53.33 g | 26.66 g | 40 g | 0.300 N | 6.00 g | v | 15.77 g | |||||
| 13 | 150 g | 66.66 g | 33.33 g | 50 g | v | v | 54.20 g | ||||||
| 14 | 150 g | 66.66 g | 33.33 g | 50 g | v | v | 19.71 g | ||||||
| 15 | 150 g | 66.66 g | 33.33 g | 50 g | 0.375 N | 7.50 g | v | 54.20 g | |||||
| 16 | 150 g | 66.66 g | 33.33 g | 50 g | 0.375 N | 7.50 g | v | 19.71 g | |||||
| Variable | A (mL) | U (mL/day) | λ (day) | R2 |
|---|---|---|---|---|
| 10% TS, C/N 25 (g/g), and Non-Delignification | 7823.54 | 300.22 | 17.83 | 0.9689 |
| 10% TS, C/N 30 (g/g), and Non-Delignification | 6608.13 | 236.07 | 19.03 | 0.9557 |
| 10% TS, C/N 25 (g/g), and Delignification | 6448.21 | 260.38 | 16.82 | 0.9449 |
| 10% TS, C/N 30 (g/g), and Delignification | 8575.42 | 320.17 | 18.27 | 0.9875 |
| 15% TS, C/N 25 (g/g), and Non-Delignification | 7647.23 | 294.75 | 17.70 | 0.9559 |
| 15% TS, C/N 30 (g/g), and Non-Delignification | 6696.53 | 237.59 | 19.54 | 0.9597 |
| 15% TS, C/N 25 (g/g), and Delignification | 7238.49 | 311.40 | 20.50 | 0.9486 |
| 15% TS, C/N 30 (g/g), and Delignification | 8967.90 | 313.33 | 19.48 | 0.9674 |
| 20% TS, C/N 25 (g/g), and Non-Delignification | 7233.81 | 256.85 | 18.97 | 0.9640 |
| 20% TS, C/N 30 (g/g), and Non-Delignification | 6422.22 | 216.06 | 19.94 | 0.9743 |
| 20% TS, C/N 25 (g/g), and Delignification | 11,161.23 | 367.71 | 23.28 | 0.9941 |
| 20% TS, C/N 30 (g/g), and Delignification | 6544.53 | 272.04 | 19.84 | 0.9622 |
| 25% TS, C/N 25 (g/g), and Non-Delignification | 9640.50 | 351.11 | 20.38 | 0.9909 |
| 25% TS, C/N 30 (g/g), and Non-Delignification | 10,610.15 | 296.07 | 24.33 | 0.9949 |
| 25% TS, C/N 25 (g/g), and Delignification | 13,498.64 | 419.38 | 23.09 | 0.9961 |
| 25% TS, C/N 30 (g/g), and Delignification | 9412.47 | 281.06 | 22.74 | 0.9668 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumardiono, S.; Jos, B.; Dewanti, A.A.E.; Mahendra, I.; Cahyono, H. Biogas Production from Coffee Pulp and Chicken Feathers Using Liquid- and Solid-State Anaerobic Digestions. Energies 2021, 14, 4664. https://doi.org/10.3390/en14154664
Sumardiono S, Jos B, Dewanti AAE, Mahendra I, Cahyono H. Biogas Production from Coffee Pulp and Chicken Feathers Using Liquid- and Solid-State Anaerobic Digestions. Energies. 2021; 14(15):4664. https://doi.org/10.3390/en14154664
Chicago/Turabian StyleSumardiono, Siswo, Bakti Jos, Agata Advensia Eksa Dewanti, Isa Mahendra, and Heri Cahyono. 2021. "Biogas Production from Coffee Pulp and Chicken Feathers Using Liquid- and Solid-State Anaerobic Digestions" Energies 14, no. 15: 4664. https://doi.org/10.3390/en14154664