Leaching of Chlorides, Sulphates, and Phosphates from Ashes Formed as a Result of Burning Conventional Fuels, Alternative Fuels, and Municipal Waste in Household Furnaces
Abstract
:1. Introduction
- (i)
- Determining the relationship between the amount of ash generated and different groups of waste and fuels used in the combustion process;
- (ii)
- Determining the content of Cl−, SO42−, and PO43− in ashes generated from the combustion of fuels and municipal waste;
- (iii)
- Comparing the correlations between the content of anions in different groups of fuels and waste;
- (iv)
- Indicating possibilities or lack thereof to reuse ashes in light of the parameters analysed.
2. Materials and Methods
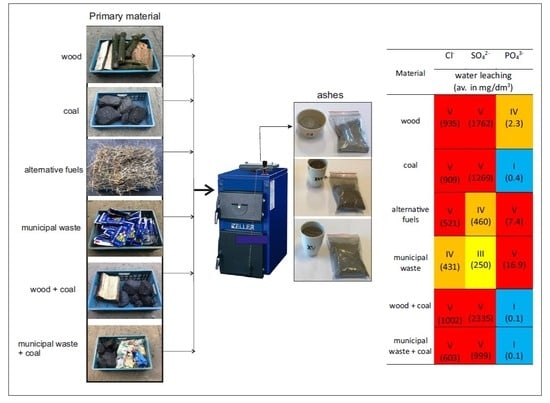
2.1. Material Preparation
- −
- Hard coal (n = 4) from various fuel storage sites: Małopolska (coal I), Mazowsze (coal II), Podkarpacie (coal III), and a sample of the so-called coal pellets,
- −
- Alternative fuels (n = 4), namely straw, peat, walnuts, and sawdust,
- −
- Wood (n = 4), representing the following species: Fraxinus (ash), Sambucus nigra L. (black elder), Acacia Mill. (acacia), and Salix L. (willow); all species were obtained from the local tree stand logging,
- −
- Municipal waste (n = 7), comprising the following fractions: paper, textiles, PET, plywood, plastic-coated paper cartons, diapers, and mixed waste (mix). Single-family household waste was collected separately and collectively.
2.2. Ash Sample Preparation
- −
- Wood alone (ash, black elder, acacia, willow, and a mix of all 4 species)—5 ash samples in total,
- −
- Hard coal alone (coal I, coal II, coal III, and coal pellets)—4 ash samples in total,
- −
- Alternative fuels alone (straw, peat, walnuts, and sawdust)—4 ash samples in total,
- −
- Municipal waste alone (paper and cardboard, plywood, plastic-coated paper cartons, and diapers)—4 ash samples in total,
- −
- Mix of wood and hard coal (ash + coal III, willow + coal I, acacia + coal III)—3 ash samples in total,
- −
- Mix of municipal waste and hard coal (textiles + coal I, PETs + coal I, and dry waste fraction mix + coal III)—3 ash samples in total.
2.3. Determination of Physical and Chemical Parameters
2.3.1. Constant Weight of Ashes
2.3.2. Preparation of Aqueous Extracts
2.3.3. Determination of Cl Content
2.3.4. Determination of SO42 Content
2.3.5. Determination of PO43− Content
3. Results and Discussion
3.1. The Amount of Ashes and the Type of Fuel and Waste Used in the Combustion Process
- −
- Dark-coloured ashes were accompanied with a large amount of non-combusted material,
- −
- Out of the wood species analysed, acacia displayed the best macroscopic parameters of ash, which means that little ash of light colour with a small amount of non-combusted material was generated,
- −
- Out of the three types of coal combusted, coal I produced the smallest amount of ash and non-combusted material (despite having the largest fraction), which points to the high quality of this coal and a small amount of mineral substance in its composition,
- −
- The addition of coal to wood results in a higher combustion temperature, and thus more effective material combustion; hence, a smaller amount of ash generated.
- −
- Combustion time affected the amount of slag and ash generated. The longer the material stayed in the furnace chamber, the more efficiently it burnt, leaving less combustion products;
- −
- Combustion temperature and rate varied greatly depending on the content of flammable substances;
- −
- Excessive air supply accelerated combustion, but at the same time, it left certain amounts of material unburnt;
- −
- Fragmented material burnt relatively faster, but at lower temperatures than large-fraction material.
3.2. Environmental Threats and Possibilities of Ash Reuse
3.2.1. Cl−
3.2.2. SO42−
3.2.3. PO43−
- Does not require the content of soluble phosphorus compounds to be determined in the case of ashes obtained after burning coal only, or
- Sets the maximum limit of 100 mg/kg in the case of ashes generated via co-combustion only.
3.3. Correlations between Anions
3.4. Verification of Research Hypotheses
Hypothesis 1 (H1). Does the addition of municipal waste (segregated and/or non-segregated) to conventional and alternative energy fuels have a significant effect on the content of chlorides, sulphates, and phosphates in ashes generated from their combustion?
Hypothesis 2 (H2). Does burning municipal waste in household furnaces significantly increase the environmental risk related to the content of chlorides, sulphates, and phosphates is ashes?
4. Conclusions
- A large amount of ash is generated after the combustion of willow wood, the mix of different types of wood, and straw. Burning of municipal waste, selected fractions, i.e., plastic-coated paper cartons and diapers, and the non-segregated fraction also produced a large amount of ash at the bottom of the furnace.
- A large amount of non-combusted material was obtained for some wood species (e.g., ash), straw, sawdust, and several municipal waste fractions, namely plastic-coated paper cartons and diapers.
- The addition of municipal waste to the process of burning fuels in household furnaces did not significantly affect the increase in chloride, sulphate, and phosphate content in ashes, the exception being diapers and plywood.
- The amounts of anions leached in ash aqueous extracts decrease in the following order:
- Cl− ash from: mix of wood and coals > wood (different species) > municipal waste > alternative fuels > mix of municipal waste and coals > coals.
- SO42− ash from: mix of wood and coals > wood (different species) > coals > mix of municipal waste and coals > alternative fuels > municipal waste.
- PO43− ash from: alternative fuels > wood (different species) > municipal waste > coals > mix of wood and coals > mix of municipal waste and coals.
- The analysis of ashes in terms of their reuse (as an additive to concrete) showed that the ashes from diapers did not meet the requirement set out in the standard, as they exceeded the allowable content of PO43− (>100 mg/kg); nor did the ashes from the mixed municipal waste fraction and coal due to the high content of Cl− (>0.1 wt %).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kicińska, A. Chemical and mineral composition of fly ashes from home furnaces, and health and environmental risk related to their presence in the environment. Chemosphere 2019, 215, 574–585. [Google Scholar] [CrossRef]
- Kicińska, A.; Mamak, M. Health risks associated with municipal waste combustion on the example of Laskowa commune (Southern Poland). Hum. Ecol. Risk Assess. Int. J. 2017, 23, 2087–2096. [Google Scholar] [CrossRef]
- Barbosa, R.; Lapa, N.; Boavida, D.; Lopes, H.; Gulyurtlu, I.; Mendes, B. Co-combustion of coal and sewage sludge: Chemical and ecotoxicological properties of ashes. J. Hazard. Mater. 2009, 170, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Brancolia, P.; Boltona, K.; Eriksson, M. Environmental impacts of waste management and valorisation pathways for surplus bread in Sweden. Waste Manag. 2020, 117, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Galvín, A.P.; Ayuso, J.; García, I.; Jiménez, J.R.; Gutiérrez, F. The effect of compaction on the leaching and pollutant emission time of recycled aggregates from construction and demolition waste. J. Clean. Prod. 2014, 83, 294–304. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ponti, J.; Valsesia, A.; La Spina, R.; Zacco, A.; Bontempi, E. Zero-waste approach in municipal solid waste incineration: Reuse of bottom ash to stabilize fly ash. J. Clean. Prod. 2020, 245, 118779. [Google Scholar] [CrossRef]
- Hjelmar, O. Leachate from land disposal of coal fly ash. Waste Manag. Res. 1990, 8, 429–449. [Google Scholar] [CrossRef]
- Saca, N.; Dimache, A.; Radu, L.R.; Iancu, I. Leaching behavior of some demolition wastes. J. Mater. Cycles Waste Manag. 2015, 19, 623–630. [Google Scholar] [CrossRef]
- Twardowska, I.; Szczepanska, J. Solid waste: Terminological and long-term environmental risk assessment problems exempli-fied in a power plant fly ash study. Sci. Total Environ. 2002, 285, 29–51. [Google Scholar] [CrossRef]
- Barbudo, A.; Galvin, A.P.; Agrela, F.; Ayuso, J.; Jiménez, J.R. Correlation analysis between sulphate content and leaching of sulphates in recycled aggregates from construction and demolition wastes. Waste Manag. 2012, 32, 1229–1235. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X. Leaching behaviour of elements from coal combustion fly ash: An overview. Int. J. Coal Geol. 2012, 94, 54–66. [Google Scholar] [CrossRef] [Green Version]
- Karuppiah, M.; Gupta, G. Toxicity of and metals in coal combustion ash leachate. J. Hazard. Mater. 1997, 56, 53–58. [Google Scholar] [CrossRef]
- Van Praagh, M.; Modin, H. Leaching of chloride, sulphate, heavy metals, dissolved organic carbon and phenolic organic pesticides from contaminated concrete. Waste Manag. 2016, 56, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Bassi, A.S.; Christensen, T.H.; Damgaard, A. Environmental performance of household waste management in Europe—An example of 7 countries. Waste Manag. 2017, 69, 545–557. [Google Scholar] [CrossRef] [Green Version]
- Butera, S.; Christensen, T.H.; Astrup, T.F. Composition and leaching of construction and demolition waste: Inorganic elements and organic compounds. J. Hazard. Mater. 2014, 276, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Querol, X.; Juan, R.; Lopez-Soler, A.; Fernandez-Turiel, J.; Ruiz, C.R. Mobility of trace elements from coal and combustion wastes. Fuel 1996, 75, 821–838. [Google Scholar] [CrossRef]
- Wilke, B.-M.; Riepert, F.; Koch, C.; Kühne, T. Ecotoxicological characterization of hazardous wastes. Ecotoxicol. Environ. Saf. 2008, 70, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Kanhar, A.H.; Chen, S.; Wang, F. Incineration Fly Ash and Its Treatment to Possible Utilization: A Review. Energies 2020, 13, 6681. [Google Scholar] [CrossRef]
- Horak, J.; Kuboňová, L.; Bajer, S.; Dej, M.; Hopan, F.; Krpec, K.; Ochodek, T. Composition of ashes from the combustion of solid fuels and municipal waste in households. J. Environ. Manag. 2019, 248, 109269. [Google Scholar] [CrossRef]
- Bachmaier, H.; Kuptz, D.; Hartmann, H. Wood Ashes from Grate-Fired Heat and Power Plants: Evaluation of Nutrient and Heavy Metal Contents. Sustainability 2021, 13, 5482. [Google Scholar] [CrossRef]
- Link, S.; Yrjas, P.; Hupa, L. Ash melting behaviour of wheat straw blends with wood and reed. Renew. Energy 2018, 124, 11–20. [Google Scholar] [CrossRef]
- Monedero, E.; Hernández, J.J.; Collado, R. Combustion-Related Properties of Poplar, Willow and Black Locust to be used as Fuels in Power Plants. Energies 2017, 10, 997. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Macías, J.; Terrones-Saeta, J.; Iglesias-Godino, F.; Corpas-Iglesias, F. Evaluation of Physical, Chemical, and Environmental Properties of Biomass Bottom Ash for Use as a Filler in Bituminous Mixtures. Sustainability 2021, 13, 4119. [Google Scholar] [CrossRef]
- Statistic Poland. Available online: https://stat.gov.pl/obszary-tematyczne/srodowisko-energia (accessed on 20 April 2019).
- Act of 14 December 2012 on Waste. J. Laws 2013, 21. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20130000021/T/D20130021L.pdf (accessed on 1 April 2021).
- Announcement of the Marshal of the Parliament of the Republic of Poland of 7 November 2016 on the Publication of the Uniform Text of the Act on Waste. Available online: http://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20160001987 (accessed on 1 April 2021).
- European Council. Council Decision 2003/33/EC of 19 December 2002 establishing criteria and procedures for the acceptance of waste at landfills pursuant to Article 16 and Annex II to Directive 1999/31/EC. Off. J. Eur. Commun. 2002, L11, 27–49. [Google Scholar]
- Minister of Environment, Regulation of the Minister of the Environment of 9 December 2014 on the Waste Catalogue. Off. J. 2014, 1923. Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20140001923/O/D20141923.pdf (accessed on 1 April 2021).
- Standard PN-EN 12457-4:2006 Characterization of Waste—Leaching—Compliance Testing for Leaching of Granular Waste Materials and Sludge; Polish Committee for Standardization: Warsaw, Poland, 2006.
- Querol, X.; Umana, J.C.; Alastuey, A.; Bertrana, C.; Lopez-Soler, A.; Plana, F. Extraction of water-soluble impurities from fly ash. Energy Sources 2000, 22, 733–750. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Biogeochemistry of Trace Elements; PWN: Warsaw, Poland, 1999. [Google Scholar]
- Minister of Environment, Regulation of the Minister of the Environment of 21 December 2015 on the Criteria and Method of Assessing the State of Groundwater Bodies. Off. J. 2015, 2148. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20160000085 (accessed on 1 April 2021).
- Minister of Health, Regulation of the Minister of Health of 7 December 2017 on the Quality of Water Intended for Human Consumption. Off. J. 2017, 2294. Available online: http://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20170002294 (accessed on 1 April 2021).
- The Act on Environmental Protection Law of 27 April 2001. J. Laws 2001, 627. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20010620627/U/D20010627Lj.pdf (accessed on 1 April 2021).
- Kabata-Pendias, A.; Motowicka-Terelak, T.; Piotrowska, M.; Terelak, H.; Witek, T. Assessment of soil and plant contamination with heavy metals and sulfur. In Framework Guidelines for Agriculture; IUNG: Pulawy, Poland, 1993. (In Polish) [Google Scholar]
- European Council. Council Directive 2008/98/EC of 19 November 2008 on Waste and Repealing Certain Directives. Off. J. Eur. Commun. 2008, L312/3, 99–126. [Google Scholar]
- Standard PN-EN 450-1:2012 Fly Ash for Concrete—Part 1: Definitions, Specifications and Compliance Criteria; Polish Committee for Standardization: Warsaw, Poland, 2012.
- Wasielewski, R.; Radko, T. The problem of managing waste from home furnaces. J. Ecol. Eng. 2018, 19, 36–44. [Google Scholar]
- Pietrzak, S.; Majewska, Z.; Wesołowski, P. The suitability of degree of phosphorus saturation indicator to assess the risk of losses this compound by runoff to surface water—Case study. Water Environ. Rural Areas 2016, 16, 89–98. [Google Scholar]
- Kanclerz, J.; Wiatrowska, K.; Adamska, A. Phosphorous concentration in surface water of Gorzuchowskie Lake catchment. Pol. J. Agron. 2015, 22, 10–17. [Google Scholar]
- Igras, J. Assessment of phosphorus losses from agricultural production in Poland. Fertil. Fertil. 2002, 4, 275–285. [Google Scholar]




| Samples | Primary Material Type | [Kg] | Colour of Ashes | Amount of Ashes [g] | Amount of Unburned Material [g] | ||
|---|---|---|---|---|---|---|---|
| Wood (W) | ash (Fraxinus) | 11.8 | very dark ashen | 106.6 | Medium * | 43.3 | a lot * |
| black elderberry (Sambucus nigra L.) | 12.1 | light grey | 100.1 | medium | 28.9 | little | |
| acacia (Acacia Mill.) | 13.0 | light grey | 101.4 | little | 31.2 | little | |
| willows (Salix L.) | 11.5 | dark ashen | 136.8 | a lot | 36.6 | medium | |
| acacia + elderberry + ash + willows | 12.2 | light grey | 111.8 | a lot | 35.1 | medium | |
| Coal (C) | coal (I) | 22.1 | light grey | 1850.1 | little | 630.2 | little |
| coal (II) | 21.5 | light grey | 4080.5 | a lot | 590.9 | little | |
| coal (III) | 22.8 | light grey | 2330.2 | medium | 593.6 | little | |
| coal pellets (ekogroszek) | 18.9 | light grey | 1812.2 | little | 460.1 | little | |
| Alternative Fuels (AF) | straw | 0.8 | very dark ashen | 113.3 | a lot | 53.7 | a lot |
| peat | 2.8 | light grey | 127.1 | medium | 48.1 | medium | |
| nuts | 1.5 | light grey | 102.2 | little | 45.5 | little | |
| sawdust | 1.2 | very dark ashen | 107.3 | little | 52.1 | a lot | |
| Municipal Waste (MW) | paper and cardboard | 1.1 | light grey | 124.3 | medium | 5.3 | little |
| plywood | 3.7 | dark ashen | 221.9 | medium | 37.6 | little | |
| tetrapaks | 0.7 | light grey | 127.5 | a lot | 907.3 | a lot | |
| diapers | 2.2 | very dark | 126.9 | a lot | 1230.0 | a lot | |
| Wood + Coal (W + C) | ash + coal (III) | 15.6 | light grey | 410.1 | little | 274.9 | little |
| willows + coal (I) | 16.2 | light grey | 450.3 | medium | 288.6 | little | |
| acacia + coal (III) | 16.1 | light grey | 400.9 | little | 267.1 | little | |
| Municipal Waste + Coal (MW + C) | fabrics + coal (I) | 13.1 | dark ashen | 520.7 | medium | 310.7 | medium |
| mix of municipal waste + coal (III) | 15.1 | light grey | 581.7 | a lot | 350.1 | medium | |
| PET drink bottle + coal (I) | 12.3 | very dark ashen | 580.3 | a lot | 323.3 | medium | |
| Sample | Cl− [mg/dm3] | Cl− [mg/kg] | ||||
|---|---|---|---|---|---|---|
| wood | ash | IV a | 404 | 934.7 b ± 464.8 c 985.8 d | 4039 | 9347.2 b ± 4648.1 c 9858.7 d |
| black elderberry | V | 510 | 5109 | |||
| acacia | V | 985 | 9857 | |||
| willows | V | 1064 | 10,642 | |||
| acacia + elderberry + ash + willows | V | 1709 | 1709 | |||
| coal | coal (I) | II | 128 | 909.5 ± 1243.9 276.6 | 1277 | 9095.1 ± 12 439.4 2766.5 |
| coal (II) | IV | 425 | 42,585 | |||
| coal (III) | V | 304 | 30,485 | |||
| coal pellets (ekogroszek) | I | 35 | 354 | |||
| alternative fuels | straw | V | 1262 | 521.3 ± 459.4 400.7 | 12,629 | 5213.7 ± 4594.2 4007.4 |
| peat | IV | 312 | 7866 | |||
| nuts | IV | 489 | 4901 | |||
| sawdust | I | 21 | 1063 | |||
| municipal waste | paper and cardboard | IV | 354 | 430.8 ± 181.1 468.0 | 3541 | 4308.5 ± 1811.6 1810.9 |
| plywood | V | 617 | 6164 | |||
| tetrapaks | III | 170 | 1701 | |||
| diapers | V | 581 | 29,118 | |||
| wood + coal | ash + coal (III) | V | 1000 | 1002.3 ± 489.3 1000.3 | 9992 | 1023.7 ± 4893.3 4893.7 |
| willows + coal (I) | IV | 404 | 4043 | |||
| acacia + coal (III) | V | 1603 | 16,031 | |||
| municipal waste + coal | fabrics + coal (I) | IV | 362 | 602.8 ± 402.7 361.7 | 3615 | 6028.2 ± 4027.1 3617.0 |
| mix of municipal waste + coal (III) | V | 1170 | 11,688 | |||
| PET drink bottle + coal (I) | IV | 276 | 2763 | |||
| for all ash samples (n = 23) | ||||||
| Min.–Max. | 21.3–3049.6 | 213.8–30 496.1 | ||||
| Av. ± SD | 736.3 ± 693.8 | 7363.5 ± 6938.4 | ||||
| Me | 489.3 | 4893.8 | ||||
| V [%] | 94.2 | 94.7 | ||||
| Sample | SO4 [mg/dm3] | SO4 [mg/kg] | ||||
|---|---|---|---|---|---|---|
| wood | ash | V a | 848 | 1762.1 b ± 616.1 c 1678.4 d | 16,946 | 35,168.3 b ± 12,313.9 c 33,509.5 d |
| black elderberry | V | 1678 | 33,509 | |||
| acacia | V | 1400 | 27,858 | |||
| willows | V | 2455 | 49,125 | |||
| acacia + elderberry + ash + willows | V | 2428 | 48,402 | |||
| coal | coal (I) | V | 1056 | 1269.5 ± 383.7 1457.3 | 21,118 | 25,329.2 ± 7646.4 29,106.8 |
| coal (II) | V | 1793 | 35,712 | |||
| coal (III) | V | 1457 | 29,107 | |||
| coal pellets (ekogroszek) | V | 772 | 15,379 | |||
| alternative fuels | straw | V | 894 | 460.5 ± 316.1 450.0 | 17,867 | 9225.1 ± 6308.6 9035.1 |
| peat | IV | 307 | 6190 | |||
| nuts | V | 593 | 11,879 | |||
| sawdust | I | 48 | 963 | |||
| municipal waste | paper and cardboard | I | 21 | 250.5 ± 265.8 163.3 | 428 | 4995.0 ± 5300.8 3253.7 |
| plywood | V | 663 | 13,228 | |||
| tetrapaks | I | 12 | 244 | |||
| diapers | IV | 305 | 6079 | |||
| wood + coal | ash + coal (III) | V | 1967 | 2335.5 ± 511.8 1980.1 | 39,296 | 46,630.9 ± 10,266.6 39,446.4 |
| willows + coal (I) | V | 3059 | 61,149 | |||
| acacia + coal (III) | V | 1980 | 39,446 | |||
| municipal waste + coal | fabrics + coal (I) | V | 1163 | 999.5 ± 254.2 1163.4 | 23,308 | 20,006.7 ± 5105.7 23,308.3 |
| mix of municipal waste + coal (III) | V | 1195 | 23,917 | |||
| PET drink bottle + coal (I) | V | 641 | 12,794 | |||
| for all samples (n = 23) | ||||||
| Min.–Max. | 12.2–3059.2 | 244.1–61,149.8 | ||||
| Av. ± SD | 1162.6 ± 839.7 | 23,215.3 ± 16,762.8 | ||||
| Me | 1055.7 | 21,118.3 | ||||
| V [%] | 72.2 | 72.2 | ||||
| Sample | PO4 [mg/dm3] | PO4 [mg/kg] | ||||
|---|---|---|---|---|---|---|
| wood | ash | V a | 5.8 | 2.3 b ± 2.0 c 1.2 d | 116.8 | 45.6 b ± 40.8 c 23.0 d |
| black elderberry | IV | 3.2 | 63.7 | |||
| acacia | IV | 1.1 | 22.1 | |||
| willows | I | 0.1 | 2.7 | |||
| acacia + elderberry + ash + willows | IV | 1.2 | 23.0 | |||
| coal | coal (I) | I | 0.1 | 0.4 ± 0.5 0.1 | 3.0 | 8.0 ± 10.1 3.0 |
| coal (II) | I | 0.1 | 2.5 | |||
| coal (III) | IV | 1.2 | 2.8 | |||
| coal (ekogroszek) | I | 0.1 | 23.8 | |||
| alternative fuels | straw | IV | 4.9 | 7.4 ± 7.1 5.1 | 98.6 | 149.0 ± 142.9 101.4 |
| peat | I | 0.3 | 6.1 | |||
| nuts | V | 19.3 | 387.2 | |||
| sawdust | V | 5.2 | 104.2 | |||
| municipal waste | paper and cardboard | I | 0.1 | 16.9 ± 29.0 0.3 | 1.7 | 337.5 ± 578.5 5.3 |
| plywood | I | 0.4 | 8.8 | |||
| tetrapaks | I | 0.01 | 0.1 | |||
| diapers | V | 67.2 | 1339.4 | |||
| wood + coal | ash + coal (III) | I | 0.2 | 0.1 ± 0.1 0.1 | 4.3 | 2.5 ± 1.3 2.2 |
| willows + coal (I) | I | 0.1 | 2.2 | |||
| acacia + coal (III) | I | 0.1 | 1.1 | |||
| municipal waste + coal | fabrics + coal (I) | I | 0.2 | 0.1 ± 0.01 0.1 | 3.0 | 1.8 ± 0.9 1.3 |
| mix of municipal waste + coal (III) | I | 0.1 | 1.3 | |||
| PET + coal (I) | I | 0.1 | 1.1 | |||
| for all samples (n = 23) | ||||||
| Min.–Max. | 0.01–67.22 | 0.1–1339.4 | ||||
| Av. ± SD | 4.8 ± 14.2 | 96.5 ± 283.6 | ||||
| Me | 0.2 | 4.3 | ||||
| V [%] | 294.4 | 293.9 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kicińska, A.; Caba, G. Leaching of Chlorides, Sulphates, and Phosphates from Ashes Formed as a Result of Burning Conventional Fuels, Alternative Fuels, and Municipal Waste in Household Furnaces. Energies 2021, 14, 3936. https://doi.org/10.3390/en14133936
Kicińska A, Caba G. Leaching of Chlorides, Sulphates, and Phosphates from Ashes Formed as a Result of Burning Conventional Fuels, Alternative Fuels, and Municipal Waste in Household Furnaces. Energies. 2021; 14(13):3936. https://doi.org/10.3390/en14133936
Chicago/Turabian StyleKicińska, Alicja, and Grzegorz Caba. 2021. "Leaching of Chlorides, Sulphates, and Phosphates from Ashes Formed as a Result of Burning Conventional Fuels, Alternative Fuels, and Municipal Waste in Household Furnaces" Energies 14, no. 13: 3936. https://doi.org/10.3390/en14133936