Advanced and Intensified Seawater Flue Gas Desulfurization Processes: Recent Developments and Improvements
Abstract
:1. Introduction
2. Land-Based SWFGD Systems
2.1. Process Description
2.2. New Developments
3. Marine SWFGD Systems
3.1. Characteristics of Marine SWFGD Process
3.2. Process Description
3.3. New Developments
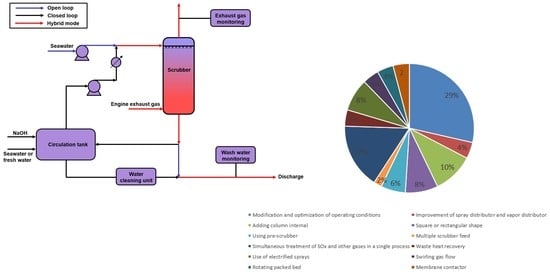
4. Improvement of Water and SWFGD Systems
4.1. Modification and Optimization of Operating Conditions
4.2. Improvement of Spray and Vapor Distributors
4.3. Adding Column Internals
4.4. Square or Rectangular Shape
4.5. Using Pre-Scrubber
4.6. Multiple Scrubber Feed
4.7. Simultaneous Treatment of SOx and Other Gases in a Single Process
4.8. Waste Heat Recovery
4.9. Use of Electrified Sprays
4.10. Swirling Gas Flow
4.11. Rotating Packed Bed
4.12. Membrane Contactor
5. Recently Commercialized Advanced and Intensified SWFGD Systems
6. Engineering Issues
7. Environmental Impacts
8. Challenges and Future Perspectives
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Soud, H.N.; Wu, Z. East Asia—Air Pollution Control and Coal-Fired Power Generation; IEACCC/06; International Energy Agency (IEA), IEA Coal Research: London, UK, 1998. [Google Scholar]
- Oikawa, K.; Yongsiri, C.; Takeda, K.; Harimoto, T. Seawater flue gas desulfurization: Its technical implications and performance results. Environ. Prog. 2003, 22, 67–73. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, L. One-dimensional simulation of synergistic desulfurization and denitrification processes for electrostatic precipitators based on a fluid-chemical reaction hybrid model. Energies 2018, 11, 3249. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, R.F.; Haglind, F.; Larsen, U. Design and modeling of an advanced marine machinery system including waste heat recovery and removal of sulphur oxides. Energy Convers. Manag. 2014, 85, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Wan, Z.; Zhu, M.; Chen, S.; Sperling, D. Three steps to a green shipping industry. Nature 2016, 530, 275–277. [Google Scholar] [CrossRef]
- Flagiello, D.; Parisi, A.; Lancia, A.; Carotenuto, C.; Erto, A.; Di Natale, F. Seawater desulphurization scrubbing in spray and packed columns for a 4.35 MW marine diesel engine. Chem. Eng. Res. Des. 2019, 148, 56–67. [Google Scholar] [CrossRef]
- Sasaki, R.; Nagayasu, T.; Shingu, T.; Watanabe, Y.; Mori, T.; Sakurai, H. Practical design of marine SOx scrubber for mega-container ships. Mitsubishi Heavy Ind. Tech. Rev. 2019, 56, 1–8. [Google Scholar]
- Córdoba, P. Status of Flue Gas Desulphurisation (FGD) systems from coal-fired power plants: Overview of the physic-chemical control processes of wet limestone FGDs. Fuel 2015, 144, 274–286. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Jozewicz, W.; Singer, C. SO2 scrubbing technologies: A review. Environ. Prog. 2001, 20, 219–228. [Google Scholar] [CrossRef]
- Srivastava, R.K. Controlling SO2 Emissions: A Review of Technologies (EPA/600/R-00/093); U.S. Environmental Protection Agency: Washington, DC, USA, 2000.
- Flagiello, D.; Erto, A.; Lancia, A.; Di Natale, F. Experimental and modelling analysis of seawater scrubbers for sulphur dioxide removal from flue-gas. Fuel 2018, 214, 254–263. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.H.; Wang, G.D.; Li, C.Y.; Zhou, J.T. Seawater flue gas desulfurization and post-desulfurization seawater recovery. Adv. Mater. Res. 2011, 233–235, 662–666. [Google Scholar] [CrossRef]
- Millero, F.J.; Hershey, P.; Johnson, G. The solubility of SO2 and the dissociation of H2SO3 in NaCl solutions. J. Atmos. Chem. 1989, 8, 377–389. [Google Scholar] [CrossRef]
- Hansen, J.P. Scrubber System and Method Technical Field. WO 2013/045272 A1, 4 April 2013. [Google Scholar]
- ENVI-MARINETM Scrubber Technology. Available online: https://maritechgroup.com/exhaust-gas-cleaning-system/#tab-1-2 (accessed on 1 April 2020).
- Abdulsattar, A.H.; Sridhar, S.; Bromley, L.A. Thermodynamics of the sulfur dioxide-seawater system. Alche J. 1977, 23, 62–68. [Google Scholar] [CrossRef]
- Rodrıguez-Sevilla, J.; Alvarez, A.; Dıaz, M.C.; Marrero, M.C. Absorption equilibria of dilute SO2 in seawater. J. Chem. Eng. 2004, 49, 1710–1716. [Google Scholar]
- Andreasen, A.; Mayer, S. Use of Seawater scrubbing for SO2 removal from marine engine exhaust gas. Energy Fuels 2007, 21, 3274–3279. [Google Scholar] [CrossRef]
- Tokumura, M.; Baba, M.; Znad, H.T.; Kawase, Y.; Yongsiri, C.; Takeda, K. Neutralization of the acidified seawater effluent from the flue gas desulfurization process: Experimental investigation, dynamic modeling, and simulation. Ind. Eng. Chem. Res. 2006, 45, 6339–6348. [Google Scholar] [CrossRef]
- Back, S.; Mojammal, A.H.M.; Jo, H.; Kim, J.; Jeong, M.; Seo, Y.; Joung, H.; Kim, S. Increasing seawater alkalinity using fly ash to restore the pH and the effect of temperature on seawater flue gas desulfurization. J. Mater. Cycles Waste Manag. 2019, 21, 962–973. [Google Scholar] [CrossRef]
- Brynolf, S.; Magnusson, M.; Fridell, E.; Andersson, K. Compliance possibilities for the future ECA regulations through the use of abatement technologies or change of fuels. Transp. Res. Part D 2014, 28, 6–18. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Elgohary, M.M. Eco-friendly selection of ship emissions reduction strategies with emphasis on SOx and NOx emissions. Int. J. Nav. Archit. Ocean Eng. 2014, 6, 737–748. [Google Scholar]
- Di Natale, F.; Carotenuto, C. Particulate matter in marine diesel engines exhausts: Emissions and control strategies. Transp. Res. Part D Transp. Environ. 2015, 40, 166–191. [Google Scholar] [CrossRef]
- EPA. Analysis of Commercial Marine Vessels Emissions and Fuel Consumption Data; EPA: Washington, DC, USA, 2000.
- Lee, S.G.; Long, N.V.D.; Lee, M. Design and optimization of natural gas liquefaction and recovery processes for offshore floating liquefied natural gas plants. Ind. Eng. Chem. Res. 2012, 51, 10021–10030. [Google Scholar] [CrossRef]
- Long, N.V.D.; Lee, M. Advances in Distillation Retrofit, 1st ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- ENVI-Marine™ Exhaust Gas Scrubbing Systems. Available online: https://www.pacificgreen-marine.com/envi-marine (accessed on 1 April 2020).
- 80% of Scrubbers are Open-Loop: DNV, GL. Available online: https://www.seatrade-maritime.com/asia/80-scrubbers-are-open-loop-dnv-gl (accessed on 1 April 2020).
- Lamas, M.I.; Rodriguez, C.G.; Rodriguez, J.D.; Telmo, J. Numerical model of SO2 scrubbing with seawater applied to marine engines. Pol. Marit. Res. 2016, 23, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Vidal, F.; Ollero, P.; Ortiz, F.J.G.; Villanueva, A. Catalytic seawater flue gas desulfurization process: An experimental pilot plant study. Environ. Sci. Technol. 2007, 41, 7114–7119. [Google Scholar] [CrossRef] [PubMed]
- Iliuta, I.; Iliuta, M.C. Modeling of SO2 seawater scrubbing in countercurrent packed-bed columns with high performance packings. Sep. Purif. Technol. 2019, 226, 162–180. [Google Scholar] [CrossRef]
- Di Natale, F.; Carotenuto, C.; Lancia, A. Enhanced SO2 removal by using charged water droplets. Chem. Eng. Trans. 2016, 52, 505–510. [Google Scholar]
- Darake, S.; Rahimi, A.; Hatamipour, M.S.; Hamzeloui, P. SO2 removal by seawater in a packed–bed tower: Experimental study and mathematical modeling. Sep. Sci. Technol. 2014, 49, 988–998. [Google Scholar] [CrossRef]
- Yeh, N.K.; Rochelle, G.T. Liquid-phase mass transfer in spray contactors. AiChE J. 2003, 49, 2363–2373. [Google Scholar] [CrossRef] [Green Version]
- Feron, P. Absorption-Based Post-Combustion Capture of Carbon Dioxide; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Bandyopadhyay, A.; Biswas, M.N. CO2 capture in a spray column using a critical flow atomizer. Sep. Purif. Technol. 2012, 94, 104–114. [Google Scholar] [CrossRef]
- Seyboth, O.; Zimmermann, S.; Heidel, B.; Scheffknecht, G. Development of a spray scrubbing process for post combustion CO2 capture with amine based solvents. Energy Procedia 2014, 63, 1667–1677. [Google Scholar] [CrossRef] [Green Version]
- Coulson, J.M.; Richardson, J.F. Chemical Engineering Volume 2, 3rd ed.; Pergamon Press: Oxford, UK, 1980. [Google Scholar]
- Javed, K.H.; Mahmud, T.; Purba, E. Enhancement of mass transfer in a spray tower using swirling gas flow. Chem. Eng. Res. Des. 2006, 84, 465–477. [Google Scholar] [CrossRef]
- Towler, G.; Sinnott, R.K. Chemical Engineering Design—Principles, Practice and Economics of Plant and Process Design, Butterworth-Heinemann; Elsevier: Burlington, MA, USA, 2013. [Google Scholar]
- Jaaskelainen, H. Engine Exhaust Back Pressure. Diesel Net Technology Guide. 2007. Available online: https://dieselnet.com/tech/diesel_exh_pres.php (accessed on 1 April 2020).
- ABS Advisory on Exhaust Gas Scrubber Systems, July 2018. Available online: https://ww2.eagle.org/content/dam/eagle/advisories-and-debriefs/exhaust-gas-scrubber-systems-advisory.pdf (accessed on 1 April 2020).
- Zhang, Y.; Gao, Y.; Zhou, J. Effects of Additives on Seawater Flue Gas Desulfurization. In 2011 International Conference on Environment Science and Engineering IPCBEE; IACSIT Press: Singapore, 2011; Volume 8, pp. 119–123. [Google Scholar]
- Schmidt, B.; Stichlmair, J. Two-phase flow and mass transfer in scrubbers. Chem. Eng. Tech. 1991, 14, 162–166. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Y.; Ren, X.; Gui, S.; Zhang, G. Absorption of sulfur dioxide with sodium hydroxide solution in spray columns. Ind. Eng. Chem. Res. 2015, 54, 8670–8677. [Google Scholar] [CrossRef]
- Flagiello, D.; Natale, F.D.; Carotenuto, C.; Erto, A.; Lancia, A. Seawater desulphurization of simulated flue gas in spray and packed columns: An experimental and modelling comparison. Chem. Eng. Trans. 2018, 69, 799–804. [Google Scholar]
- Caiazzo, G.; Langella, G.; Miccio, F.; Scala, F. An experimental investigation on seawater SO2 scrubbing for marine application. Environ. Prog. Sustain. Energy 2013, 36, 1179–1186. [Google Scholar] [CrossRef]
- Zhao, X.; Hou, C.; Fan, L.; Cui, Z. Absorption of sulfur dioxide by seawater and concentrated seawater. Chin. J. Environ. Eng. 2013, 7, 3123–3130. [Google Scholar]
- Ma, Y.; Xu, L.; Su, P.; Feng, D.; Yang, K. Study on seawater scrubbing for SO2 removal from ship’s power plant exhaust gas. Environ. Prot. Eng. 2020, 46, 31–47. [Google Scholar]
- Cao, H.W.; Dong, F. Study status of additives for efficient sulfur retention in FGD. Energy Conserv. Technol. 2003, 21, 10–12. [Google Scholar]
- Wang, J.G.; Hu, J.B.; Duan, Z.Y.; Chen, Z.Q. The application of compound additives in two kinds of flue-gas desulfurization process. J. Eng. Energy Power 2006, 21, 93–95. [Google Scholar]
- Liu, S.Y.; Liu, P.; Gao, J.; Liu, J.Y.; Ye, Z.X.; Xu, C.H. Simulation studies on limestone dissolution with organic acid additives in limestone-based flue gas desulfurization. In Proceedings of the 2nd International Conference on Bioinformatics and Biomedical Engineering (ICBBE 2008), Shanghai, China, 16–18 May 2008; pp. 3899–3902. [Google Scholar]
- Dahlan, I.; Lee, K.T.; Amaruddin, A.H.; Mohamed, A.R. Evaluation of various additives on the preparation of rice husk ash (RHA)/CaO-based sorbent for flue gas desulfurization (FGD) at low temperature. J. Hazard. Mater. 2009, 161, 81–83. [Google Scholar] [CrossRef]
- Zhai, L.Z.; Zhong, Q.; He, C. Experimental research on effect of acids as additives on wet flue gas desulfurization with ethylenediamine. In Proceedings of the International Conference on Energy and Environment Technology (ICEET ’09), Guilin, China, 16–18 October 2009; pp. 327–331. [Google Scholar]
- Chu, X.F.; Tian, J.R.; Sun, B.H.; Chen, G.L.; Ding, F.J. Pilot research on demonstration project of desulphurization via white clay and sea-water. Environ. Eng. 2009, 27, 81–83. [Google Scholar]
- Kister, H.Z. Distillation Operation; McGraw-Hill: New York, NY, USA, 1989. [Google Scholar]
- Aly, F.A.; Yeung, S.Y. High Efficiency Radial Type Vapor Distributor for Packed Towers. U.S. Patent 4810428A, 7 March 1989. [Google Scholar]
- Bandyopadhyay, A.; Biswas, N.B. Critical flow atomizer in SO2 spray scrubbing. Chem. Eng. J. 2008, 139, 29–41. [Google Scholar] [CrossRef]
- Biswas, M.N. Atomization in two-phase critical flow. In Proceedings of the 2nd International Conference on Liquid Atomization and Spray Systems-II, Madison, WI, USA, 20–24 June 1982; pp. 145–151. [Google Scholar]
- Flagiello, D.; Di Natale, F.; Erto, A.; Lancia, A. Marine diesel engine flue gas desulphurization by seawater scrubbing in a structured packing absorption column. In Proceedings of the 40th ASICI, Rome, Italy, 7–9 June 2017. [Google Scholar]
- Schultes, M. Raschig Super-Ring—A new fourth generation packing offers new advantages. Chem. Eng. Res. Des. 2003, 81, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Just, P.E. Shell Cansolv deploying CCS worldwide. In Proceedings of the Second Post-Combustion Capture Conference (PCCC2), Bergen, Norway, 17–20 September 2013. [Google Scholar]
- How Does It work? Available online: https://www.pacificgreen-marine.com/envi-marine/how-does-it-work (accessed on 1 April 2020).
- Wang, Z.; Kuang, H.; Zhang, J.; Chu, L.; Ji, Y. Experimental study on the removal of real exhaust pollutants from a diesel engine by activated carbon. Appl. Sci. 2019, 9, 3175. [Google Scholar] [CrossRef] [Green Version]
- Peng, S. Ship Flue Gas Desulfurization Method and Equipment. U.S. Patent 8,038,774 B2, 8 October 2011. [Google Scholar]
- Kasper, A.; Aufdenblatten, S.; Forss, A.; Mohr, M.; Burtscher, H. Particulate emissions from a low-speed marine diesel engine. Aerosol Sci. Technol. 2007, 41, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Al-Enezi, G.; Ettouney, H.; El-Dessouky, H.; Fawzi, N. Solubility of sulfur dioxide in seawater. Ind. Eng. Chem. Res. 2001, 40, 1434–1441. [Google Scholar] [CrossRef]
- Qi, Y.; Hu, X.; Liu, Y.; Sun, D.; Li, R.; Zhu, H. Highly efficient and reversible absorption of SO2 by hydroxyl ammonium ionic liquids at low partial pressure. J. Chem. Technol. Biotechnol. 2019, 94, 3325–3332. [Google Scholar] [CrossRef]
- Emission Project Guide, MAN B&W Two-Stroke Marine Engines. Available online: https://indico.cern.ch/event/659434/attachments/1528657/2391369/MAN_BW_Two-stroke_Marine_Engines.pdf (accessed on 1 April 2020).
- Product Guide—SOx Scrubber Technology. Available online: https://cdn.wartsila.com/docs/default-source/product-files/egc/product-guide-o-env-sox-scrubber-tech.pdf (accessed on 1 April 2020).
- Venturi Scrubber. Available online: https://emis.vito.be/en/bat/tools-overview/sheets/venturi-scrubber (accessed on 1 April 2020).
- Polasek, J.C.; Bullin, J.A.; Donnelly, S.T. Alternative Flow Schemes to Reduce Capital and Operating Costs of Amine Sweetening Units. In Proceedings of the 1982 AIChE Spring National Meeting, New York, NY, USA, 1982; American Institute of Chemical Engineers: New York, NY, USA, 1982. [Google Scholar]
- Lyddon, L.; Nguyen, H. Analysis of various flow schemes for sweetening with amines. In Proceedings of the 78th GPA Annual Convention, Nashville, TN, USA, 2–3 March 1999; pp. 177–184. [Google Scholar]
- Cho, H.; Binns, M.; Min, K.J.; Kim, J.K. Automated process design of acid gas removal units in natural gas processing. Comp. Chem. Eng. 2015, 83, 97–109. [Google Scholar] [CrossRef]
- Zhoua, J.; Wang, H. Study on efficient removal of SOx and NOx from marine exhaust gas by wet scrubbing method using urea peroxide solution. Chem. Eng. J. 2020, 390, 124567. [Google Scholar] [CrossRef]
- Park, H.W.; Choi, S.; Park, D.W. Simultaneous treatment of NO and SO2 with aqueous NaClO2 solution in a wet scrubber combined with a plasma electrostatic precipitator. J. Hazard. Mater. 2015, 285, 117–126. [Google Scholar] [CrossRef]
- Chu, H.; Chien, T.W.; Li, S.Y. Simultaneous absorption of SO2 and NO from flue gas with KMnO4/NaOH solutions. Sci. Total Environ. 2001, 275, 127–135. [Google Scholar] [CrossRef]
- Jin, D.S.; Deshwal, B.R.; Park, Y.S.; Lee, H.K. Simultaneous removal of SO2 and NO by wet scrubbing using aqueous chlorine dioxide solution. J. Hazard. Mater. 2006, 135, 412–417. [Google Scholar] [CrossRef]
- Chien, T.W.; Chu, H.; Hsueh, H.T. Kinetic study on absorption of SO2 and NOx with acidic NaClO2 solutions using the spraying column. J. Environ. Eng. 2003, 129, 967–974. [Google Scholar] [CrossRef]
- Hutson, N.D.; Kryzynska, R.; Srivastava, R. Simultaneous removal of SO2, NOx and Hg from coal flue gas using a NaClO2–enhanced wet scrubber. Ind. Eng. Chem. Res. 2008, 47, 5825–5831. [Google Scholar] [CrossRef]
- Pillai, K.C.; Chung, S.J.; Raju, T.; Moon, I.S. Experimental aspects of combined NOx and SO2 removal from flue–gas mixture in an integrated wet scrubber-electrochemical cell system. Chemosphere 2009, 76, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Pourmohammadbagher, A.; Jamshidi, E.; Ale-Ebrahim, H.; Dabir, S. Study on simultaneous removal of NOx and SO2 with NaClO2 in a novel swirl wet system. Ind. Eng. Chem. Res. 2011, 50, 8278–8284. [Google Scholar] [CrossRef]
- Puxty, G.; Wei, S.C.; Feron, P.; Meuleman, E.; Beyad, Y.; Burns, R.; Maeder, M. A novel process concept for the capture of CO2 and SO2 using a single solvent and column. Energy Procedia 2014, 63, 703–714. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S. Process Evaluation of a SOx and NOx Exhaust Gas Cleaning Concept for Marine Application; Department of Energy and Environment, Chalmers University of Technology Gothenburg: Gothenburg, Sweden, 2016. [Google Scholar]
- Singh, D.V.; Pedersen, E. A review of waste heat recovery technologies for maritime applications. Energy Convers. Manag. 2016, 111, 315–328. [Google Scholar] [CrossRef]
- Viking Line, Viking Grace First Ship to Install New Swedish Heat Recovery System. Available online: https://www.vikingline.com/globalassets/documents/market_specific/corporate/press/pressreleaseeng/2014/20141201-climeon-eng.pdf (accessed on 14 June 2020).
- Marinelog, Maersk Vessel First with New Waste Heat Recovery System—Marine Log. Available online: http://www.marinelog.com/index.php?option=com_k2&view=item&id=10842%253Amaersk-vesselfirst-with-new-waste-heat-recovery-system&Itemid=231 (accessed on 14 June 2020).
- Ng, C.W.; Tam, I.C.K.; Wu, D. Thermo-economic performance of an organic rankine cycle system recovering waste heat onboard an offshore service vessel. J. Mar. Sci. Eng. 2020, 8, 351. [Google Scholar] [CrossRef]
- Shu, G.; Liang, Y.; Wei, H.; Tian, H.; Zhao, J.; Liu, L. A review of waste heat recovery on two-stroke IC engine aboard ships. Renew. Sustain. Energy Rev. 2012, 19, 385–401. [Google Scholar] [CrossRef]
- Green Car Congress, First Reference Installation of Opcon Waste Heat Recovery Technology for Ships; Potential for 5–10% Fuel Savings. Available online: https://www.greencarcongress.com/2012/08/opcon-20120826.html (accessed on 23 June 2020).
- Di Natale, F.; Carotenuto, C.; Manna, L.; Lancia, A. Chemi-electro-hydrodynamic of sulphur dioxide absorption by electrified water sprays. Chem. Eng. Trans. 2018, 69, 685–690. [Google Scholar]
- Di Natale, F.; Carotenuto, C.; Caserta, S.; Troiano, M.; Manna, L.; Lancia, A. Experimental evidences on the chemi-electro-hydrodynamic absorption of sulphur dioxide in electrified water sprays. Chem. Eng. Res. Des. 2019, 146, 249–262. [Google Scholar] [CrossRef]
- ANY SOx scubber system. Available online: http://www.anytech.co.kr/index.php?ver=PCver (accessed on 23 June 2020).
- Schrauwen, F.J.M.; Thoenes, D. Selective gas absorption in a cyclone spray scrubber. Chem. Eng. Sci. 1988, 43, 2189–2194. [Google Scholar] [CrossRef]
- Mallinson, R.H.; Ramshaw, C. Mass Transfer Process. U.S. Patent 4,283,255, 11 August 1981. [Google Scholar]
- Rao, D.P.; Bhowal, A.; Goswami, P.S. Process intensification in rotating packed beds (Higee): An appraisal. Ind. Eng. Chem. Res. 2004, 43, 1150–1162. [Google Scholar] [CrossRef]
- Long, N.V.D.; Minh, L.Q.; Luis, P.; Lee, M. Intensified distillation-based separation processes: Recent developments and perspectives. Chem. Eng. Technol. 2016, 39, 2183–2195. [Google Scholar] [CrossRef]
- Liangliang, Z.; Shuying, W.; Yue, G.; Baochang, S.; Yong, L.; Haikui, Z.; Guangwen, C.; Jianfeng, C. Absorption of SO2 with calcium-based solution in a rotating packed bed. Sep. Purif. Technol. 2019, 214, 148–155. [Google Scholar]
- Liu, Y.Z.; Wu, W.; Liu, Y.; Li, B.B.; Luo, Y.; Chu, G.W.; Zuo, H.W.; Chen, J.F. Desulfurization intensification by ionic liquid in a rotating packed bed. Chem. Eng. Process. 2020, 148, 107793. [Google Scholar] [CrossRef]
- Hacking, J.A.; Delsing, N.F.E.J.; de Beer, M.M.; van der Schaaf, J. Improving liquid distribution in a rotating packed bed. Chem. Eng. Process. 2020, 149, 107861. [Google Scholar] [CrossRef]
- Karoor, S.; Sirkar, K.K. Gas-absorption studies in microporous hollow fiber membrane modules. Ind. Eng. Chem. Res. 1993, 32, 674–684. [Google Scholar] [CrossRef]
- Zhang, Q.; Cussler, E.L. Microporous hollow fibers for gas-absorption. 1. Mass-transfer in the liquid. J. Membr. Sci. 1985, 23, 321–332. [Google Scholar]
- Sun, X.; Meng, F.; Yang, F. Application of seawater to enhance SO2 removal from simulated flue gas through hollow fiber membrane contactor. J. Membr. Sci. 2008, 312, 6–14. [Google Scholar] [CrossRef]
- Liu, J.; Deng, M.; Yuan, J.; Ji, Z.; Zhao, Y.; Guo, X. An aeration membrane absorption seawater flue gas desulfurization process intensified by combining dual-phase flow and oxidation reaction. Chem. Eng. Process. 2020, 153, 107935. [Google Scholar] [CrossRef]
- Fuji Electric’s First Shipment of Exhaust Gas Cleaning Systems for Ships. Available online: https://www.fujielectric-europe.com/en/fuji/news/news_detail/n/fuji_electric_s_first_shipment_of_exhaust_gas_cleaning_systems_for_ships (accessed on 1 April 2020).
- Practical Considerations for the Installation and Operation of Exhaust Gas Cleaning Systems. 2019. Available online: https://www.standard-club.com/media/3229390/abs_scrubber-guidance.pdf (accessed on 1 April 2020).
- Marine FGD Gas Scrubbing/Cleaning Nozzles. Available online: https://www.bete.com/applications/marinescrubbing (accessed on 1 April 2020).
- International Agency for Research on Cancer, 2012. IARC: Diesel Engine Exhaust Carcinogenic. Available online: https://www.iarc.fr/wp-content/uploads/2018/07/pr213_E.pdf (accessed on 1 April 2020).
- Hesketh, E. Air Pollution Control, Traditional and Hazardous Pollutants; Technomic Publishing AG: Lancaster, PA, USA, 1996. [Google Scholar]
- Poullikkas, A. Review of design, operating, and financial considerations in flue gas desulfurization systems. Energy Technol. Pol. 2015, 2, 92–103. [Google Scholar] [CrossRef]
- Silva, A.M.; Lima, R.M.F.; Leao, V.A. Mine water treatment with limestone for sulfate removal. J. Hazard. Mater. 2012, 221–222, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, P.; Tang, Z.; Chen, X.; Huang, J.; Tang, Z.; Cen, C. Removal of high-concentration sulfate ions from the sodium alkali FGD wastewater using ettringite precipitation method: Factor assessment, feasibility, and prospect. J. Chem. 2018, 2008, 1265168. [Google Scholar] [CrossRef]
- Najib, T.; Solgi, M.; Farazmand, A.; Heydarian, S.M.; Nasernejad, B. Optimization of sulfate removal by sulfate reducing bacteria using response surface methodology and heavy metal removal in a sulfidogenic UASB reactor. J. Environ. Chem. Eng. 2017, 5, 3256–3265. [Google Scholar] [CrossRef]
- Kiran, M.G.; Pakshirajan, K.; Das, G. Heavy metal removal from multicomponent system by sulfate reducing bacteria: Mechanism and cell surface characterization. J. Hazard. Mater. 2017, 324, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zou, W.; Chai, P.; Wang, J.; Bazinet, L. Feasibility of antibiotic and sulfate ions separation from wastewater using electrodialysis with ultra-filtration membrane. J. Clean. Prod. 2016, 112, 3097–3105. [Google Scholar] [CrossRef]
- Hong, S.Q.; Cannon, F.S.; Hou, P.; Byrne, T.; Nieto-Delgado, C. Adsorptive removal of sulfate from acid mine drainage by polypyrrole modified activated carbons: Effects of polypyrrole deposition protocols and activated carbon source. Chemosphere 2017, 184, 429–437. [Google Scholar] [CrossRef]
- Calinescu, O.; Marin, N.M.; Ionita, D.; Pascu, L.F.; Tudorache, A.; Surpățeanue, G.; Badea, I.A.; Aboul-Enein, H.Y. Selective removal of sulfate ion from different drinking waters. Environ. Nanotechnol. Monit. Manag. 2016, 6, 164–168. [Google Scholar] [CrossRef]
- Arahman, N.; Mulyati, S.; Lubis, M.R.; Takagi, R.; Matsuyama, H. Removal profile of sulfate ion from mix ion solution with different type and configuration of anion exchange. J. Water Process Eng. 2017, 20, 173–179. [Google Scholar] [CrossRef]
- Tait, S.; Clarke, W.P.; Keller, J.; Batstone, D.J. Removal of sulfate from high-strength wastewater by crystallization. Water Res. 2009, 43, 762–772. [Google Scholar] [CrossRef]
- Luna, M.D.G.; Rance, D.P.M.; Bellotindos, L.M.; Lu, M.C. Removal of sulfate by fluidized bed crystallization process. J. Env. Chem. Eng. 2017, 5, 2431–2439. [Google Scholar] [CrossRef]
- Benatti, C.T.; Tavares, C.R.G.; Lenzi, E. Sulfate removal from waste chemicals by precipitation. J. Environ. Manag. 2009, 90, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Runtti, H.; Luukkonen, T.; Niskanen, M.; Tuomikoski, S.; Kangas, T.; Tynjälä, P.; Tolonen, E.; Sarkkinen, M.; Kemppainen, K.; Rämö, J.; et al. Sulphate removal over barium-modified blast-furnace-slag geopolymer. J. Hazard. Mater. 2016, 317, 373–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kartic, D.N.; Narayana, B.C.A.; Arivazhagan, M. Removal of high concentration of sulfate from pigment industry effluent by chemical precipitation using barium chloride: RSM and ANN modeling approach. J. Env. Manag. 2018, 206, 69–76. [Google Scholar] [CrossRef]
- Dou, W.X.; Zhou, Z.; Jiang, L.M.; Jiang, A.; Huang, R.; Tian, X.; Zhang, W.; Chen, D. Sulfate removal from wastewater using ettringite precipitation: Magnesium ion inhibition and process optimization. J. Environ. Manag. 2017, 196, 518–526. [Google Scholar] [CrossRef]
- Dulière, V.; Baetens, K.; Lacroix, G. Potential Impact of Wash Water Effluents from Scrubbers on Water Acidification in the Southern North Sea; Royal Belgian Institute of Natural Sciences: Bruxelles, Belgium, 2020. [Google Scholar]
- Teuchies, J.; Cox, T.J.S.; Itterbeeck, K.V.; Meysman, F.J.R.; Blust, R. The impact of scrubber discharge on the water quality in estuaries and ports. Environ. Sci. Eur. 2020, 32, 103. [Google Scholar] [CrossRef]
- Turner, D.R.; Edman, M.; Gallego-Urrea, J.A.; Claremar, B.; Hassellöv, I.; Omstedt, A.; Rutgersson, A. The potential future contribution of shipping to acidification of the Baltic Sea. Ambio 2018, 47, 368–378. [Google Scholar] [CrossRef]










| Thermal Power Plants | Large Ships | |
|---|---|---|
| Flue gas flow rate (Nm3/h) | 600,000 to 4,000,000 | 23,000 to 540,000 |
| Inlet SO2 level (ppmd) | 100 to 1800 | 700 |
| Outlet SO2 level (ppmd) | 10 to 220 | 20 |
| SO2 removal efficiency (%) | 75 to 98 | 97.1 (3.5%S to 0.1%S): SECAs 85.7 (3.5%S to 0.5%S): global sea areas excluding SECAs |
| Regulatory items for seawater discharge | pH, dissolved oxygen (DO), temperature, etc. | pH, PAH, turbidity, nitrates |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, N.V.D.; Lee, D.Y.; Jin, K.M.; Choongyong, K.; Mok, L.Y.; Won, L.S.; Lee, M. Advanced and Intensified Seawater Flue Gas Desulfurization Processes: Recent Developments and Improvements. Energies 2020, 13, 5917. https://doi.org/10.3390/en13225917
Long NVD, Lee DY, Jin KM, Choongyong K, Mok LY, Won LS, Lee M. Advanced and Intensified Seawater Flue Gas Desulfurization Processes: Recent Developments and Improvements. Energies. 2020; 13(22):5917. https://doi.org/10.3390/en13225917
Chicago/Turabian StyleLong, Nguyen Van Duc, Dong Young Lee, Kim Myung Jin, Kwag Choongyong, Lee Young Mok, Lee Sung Won, and Moonyong Lee. 2020. "Advanced and Intensified Seawater Flue Gas Desulfurization Processes: Recent Developments and Improvements" Energies 13, no. 22: 5917. https://doi.org/10.3390/en13225917