CFD-DEM Simulation of Biomass Pyrolysis in Fluidized-Bed Reactor with a Multistep Kinetic Scheme
Abstract
:1. Introduction
2. Computational Model
3. Numerical Algorithm
4. Results and Discussions
4.1. Validation
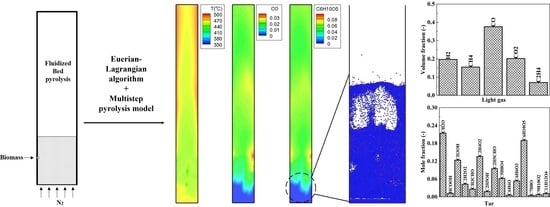
4.2. Pyrolysis Phenomena
4.3. Influence of Pyrolysis Temperature
4.4. Influence of Particle Size
5. Conclusions
- (1)
- The novelty of the integrated algorithm lies in the utilization of a multistep kinetic scheme for the detailed simulation of particle pyrolysis, thus allowing for a practical consideration of product variation with the change of operating conditions. The current work is the first step for future studies of more complex issues such as tar removal and pollutant reduction.
- (2)
- The integrated CFD-DEM and multistep pyrolysis model is validated with experimental data and is also compared with the predictions of a two-fluid model. An overall improvement is found in light gas, tar, and char yields, among which, the relative error of the predicted tar yield is reduced by more than 50%, while that of the CO and C2H4 yields is reduced by approximately 30%. In addition, fluidization behaviors, such as the biomass particle distribution and tar evolution, which cannot easily observed in experiments, are also obtained.
- (3)
- Under the specific pyrolysis conditions at hand, light gas yield increases considerably from 17% to 25% when the pyrolysis temperature rises from 500 to 700 °C. In addition, the primary tar yield stays around 46% and only shows a slight influence by the pyrolysis temperature. The variation in biomass particle size is found to have a significant impact on particle flow pattern, while pyrolysis products only show small variations within the tested range of particle diameter (<1 mm).
Author Contributions
Funding
Conflicts of Interest
References
- Scarlat, N.; Dallemand, J.; Monforti-Ferrario, F.; Banja, M.; Motola, V. Renewable energy policy framework and bioenergy contribution in the European Union—An overview from national renewable energy action plans and progress reports. Renew. Sustain. Energy Rev. 2015, 51, 969–985. [Google Scholar] [CrossRef]
- Polin, J.P.; Peterson, C.A.; Whitmer, L.E.; Smith, R.G.; Brown, R.G. Process intensification of biomass fast pyrolysis through autothermal operation of a fluidized-bed reactor. Appl. Energy 2019, 249, 276–285. [Google Scholar] [CrossRef]
- Cai, W.; Liu, R.; He, Y.; Chai, M.; Cai, J. Bio-oil production from fast pyrolysis of rice husk in a commercial-scale plant with a downdraft circulating fluidized-bed reactor. Fuel Process. Technol. 2018, 171, 308–317. [Google Scholar] [CrossRef]
- Blanco, A.; Chejne, F. Modeling and simulation of biomass fast pyrolysis in a fluidized-bed reactor. J. Anal. Appl. Pyrolysis 2016, 118, 105–114. [Google Scholar] [CrossRef]
- Molino, A.; Lovane, P.; Donatelli, A.; Braccio, G.; Chianese, S.; Musmarra, D. Steam gasification of resuse-derived fuel in a rotary kiln pilot plant: Experimental tests. Chem. Eng. Trans. 2013, 32, 337–342. [Google Scholar]
- Victor, B.A.; Abdullah, T.A.R.B.T.; Alsaffar, M.A.; Mustapa, S.I.; Salleh, S.F. Recent advances in renewable hydrogen production by thermo-catalytic conversion of biomass-derived glycerol: Overview of prospects and challenges. Int. J. Hydrogen Energy 2020, 45, 18160–18185. [Google Scholar]
- Mudhoo, A.; Torres-Mayanga, P.C.; Forster-Carneiro, T.; Sivagurunathan, P.; Kumar, G.; Komilis, D.; Sánchez, A. A review of research trends in the enhancement of biomass-to-hydrogen conversion. Waste Manag. 2018, 79, 580–594. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Manovic, V.; Hanak, D.P. Techno-economic assessment of coal- or biomass-fired oxy-combustion power plants with supercritical carbon dioxide cycle. Energy Convers. Manag. 2020, 221, 113143. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Q.; Shao, Y.; Zhong, W. Energy and exergy analysis of oxy-fuel combustion based on circulating fluidized bed power plant firing coal, lignite and biomass. Fuel 2020, 269, 117424. [Google Scholar] [CrossRef]
- Zhang, Y.; Chao, Z.; Jakobsen, H.A. Modeling and simulation of chemical looping combustion process in a double loop circulating fluidized reactor. Chem. Eng. J. 2017, 320, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fang, Y.; Jin, B.; Zhang, Y.; Zhou, C.; Sher, F. Effect of slot wall jet on combustion process in a 660MW opposed wall fired pulverized coal Boiler. Int. J. Chem. React. Eng. 2019, 17. [Google Scholar] [CrossRef]
- Pérez, S.; Del Molino, E.D.; Barrio, V.L. Modeling and testing of a milli-structured reactor for carbon dioxide methanation. Int. J. Chem. React. Eng. 2019, 20180238. [Google Scholar] [CrossRef]
- Sher, F.; Iqbal, S.Z.; Albazzaz, S.; Ali, U.; Mortari, D.A.; Rashid, T. Development of biomass derived highly porous fast adsorbents for post-combustion CO2 capture. Fuel 2020, 282, 118506. [Google Scholar] [CrossRef]
- Sher, F.; Yaqoob, A.; Saeed, F.; Zhang, S.; Jahan, Z.; Klemeš, J.J. Torrefied biomass fuels as a renewable alternative to coal in co-firing for power generation. Energy 2020, 209, 118444. [Google Scholar] [CrossRef]
- Mallick, D.; Mahanta, P.; Moholkar, V.S. Co-gasification of coal and biomass blends: Chemistry and engineering. Fuel 2017, 204, 106–128. [Google Scholar] [CrossRef]
- Sher, F.; Iqbal, S.Z.; Liu, H.; Imran, M.; Snape, C.E. Thermal and kinetic analysis of diverse biomass fuels under different reaction environment: A way forward to renewable energy sources. Energy Convers. Manag. 2020, 203, 112266. [Google Scholar] [CrossRef]
- Seddighi, S.; Clough, P.T.; Anthony, E.J.; Hughes, R.W.; Lu, P. Scale-up challenges and opportunities for carbon capture by oxy-fuel circulating fluidized-beds. Appl. Energy 2018, 232, 527–542. [Google Scholar] [CrossRef]
- Dingh, R.I.; Kumar, R. Current status and experimental investigation of oxy-fired fluidized-bed. Renew. Sustain. Energy Rev. 2016, 61, 398–420. [Google Scholar]
- Hai, I.U.; Sher, F.; Zarren, G.; Liu, H. Experimental investigation of tar arresting techniques and their evaluation for product syngas cleaning from bubbling fluidized bed gasifier. J. Clean. Prod. 2019, 240, 118239. [Google Scholar] [CrossRef]
- Williams, A.; Jones, J.M.; Ma, L.; Pourkashanian, M. Pollutants from the combustion of solid biomass fuels. Prog. Energy Combust. Sci. 2012, 38, 113–137. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Hameed, S.; Sharma, A.; Pareek, V.; Wu, H.; Yu, Y. A review on biomass pyrolysis models: Kinetic, network and mechanistic models. Biomass Bioenergy 2019, 123, 104–122. [Google Scholar] [CrossRef]
- Prakash, N.; Karunanithi, T. Kinetic modeling in biomass pyrolysis—A review. J. Appl. Sci. Res. 2008, 4, 1627–1636. [Google Scholar]
- Gerber, S.; Behrendt, F.; Oevermann, M. An Eulerian modeling approach of wood gasification in a bubbling fluidized-bed reactor using char as bed material. Fuel 2010, 89, 2903–2917. [Google Scholar] [CrossRef]
- Aghaalikhani, A.; Schmid, J.C.; Borello, D.; Fuchs, J.; Benedikt, F.; Hofbauer, H.; Rispoli, F.; Henriksen, U.B.; Sárossy, Z.; Cedola, L. Detailed modeling of biomass steam gasification in a dual fluidized-bed gasifier with temperature variation. Renew. Energy 2019, 143, 703–718. [Google Scholar] [CrossRef]
- Wang, S.; Luo, K.; Hu, C.; Sun, L.; Fan, J. Impact of operating parameters on biomass gasification in a fluidized-bed reactor: An Eulerian-Lagrangian approach. Powder Technol. 2018, 333, 304–316. [Google Scholar] [CrossRef]
- Miller, R.S.; Bellan, J. A generalized biomass pyrolysis model based on superimposed cellulose, hemicellulose and lignin kinetics. Combust. Sci. Technol. 1997, 126, 97–137. [Google Scholar] [CrossRef]
- Niksa, S. Predicting the rapid devolatilization of diverse forms of biomass with bio-flashchain. Proc. Combust. Inst. 2000, 28, 2727–2733. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.; Zhao, X.; Cao, J. Biomass thermochemical conversion: A review on tar elimination from biomass catalytic gasification. J. Energy Inst. 2020, 93, 1083–1098. [Google Scholar] [CrossRef]
- Thangalazhy-Gopakumar, S.; Adhikari, S.; Gupta, R.B.; Fernando, S.D. Influence of pyrolysis operating conditions on bio-oil components: A microscale study in a pyroprobe. Energy Fuels 2011, 25, 1191–1199. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K. A review on the pyrolysis of woody biomass to bio-oil: Focus on kinetic models. Renew. Sustain. Energy Rev. 2015, 52, 1580–1595. [Google Scholar] [CrossRef]
- Islam, M.W. A review of dolomite catalyst for biomass gasification tar removal. Fuel 2020, 267, 117095. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Rosen, M.A.; Tyagi, S.K. Global challenges in the sustainable development of biomass gasification: An overview. Renew. Sustain. Energy Rev. 2017, 80, 23–43. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, Z.; Zhang, Z.; Guo, C.; Ellis, N.; Bi, X. Tar elimination from biomass gasification syngas with bauxite residue derived catalyst and gasification char. Appl. Energy 2020, 258, 114088. [Google Scholar] [CrossRef]
- Ranzi, E.; Cuoci, A.; Faravelli, T.; Frassoldati, A.; Migliavacca, G.; Pierucci, S.; Sommariva, S. Chemical kinetics of biomass pyrolysis. Energy Fuels 2008, 22, 4292–4300. [Google Scholar] [CrossRef]
- Debiagi, P.E.A.; Pecchi, C.; Gentile, G.; Frassoldati, A.; Cuoci, A.; Faravelli, T.; Ranzi, E. Extractives extend the applicability of multistep kinetic scheme of biomass pyrolysis. Energy Fuels 2015, 29, 6544–6555. [Google Scholar] [CrossRef]
- Gentile, G.; Debiagi, P.E.A.; Cuoci, A.; Frassoldati, A.; Ranzi, E.; Faravelli, T. A computational framework for the pyrolysis of anisotropic biomass particles. Chem. Eng. J. 2017, 321, 458–473. [Google Scholar] [CrossRef]
- Chen, T.; Ku, X.; Lin, J.; Ström, H. Pyrolysis simulation of thermally thick biomass particles based on a multistep kinetic scheme. Energy Fuels 2020, 34, 1940–1957. [Google Scholar] [CrossRef]
- Sommariva, S.; Grana, R.; Maffei, T.; Pierucci, S.; Ranzi, E. A kinetic approach to the mathematical model of fixed bed gasifiers. Comput. Chem. Eng. 2011, 35, 928–935. [Google Scholar] [CrossRef]
- Peng, J.; Eri, Q.; Zhao, X. Detailed simulations of fast pyrolysis of biomass in a fluidized-bed reactor. J. Renew. Sustain. Energy 2018, 10, 013104. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Ronsse, F.; Nachenius, R.; Pieters, J.G. 3D Eulerian-Eulerian modeling of a screw reactor for biomass thermochemical conversion. Part 2: Slow pyrolysis for char production. Renew. Energy 2019, 143, 1477–1487. [Google Scholar] [CrossRef]
- Ranganathan, P.; Gu, S. Computational fluid dynamics modeling of biomass fast pyrolysis in fluidized-bed reactors, focusing different kinetic schemes. Bioresour. Technol. 2016, 213, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranzi, E.; Corbetta, M.; Manenti, F.; Pierucci, S. Kinetic modeling of the thermal degradation and combustion of biomass. Chem. Eng. Sci. 2014, 110, 2–12. [Google Scholar] [CrossRef]
- Xiong, Q.; Yang, Y.; Xu, F.; Pan, Y.; Zhang, J.; Hong, K.; Lorenzini, G.; Wang, S. Overview of computational fluid dynamics simulation of reactor-scale biomass pyrolysis. ACS Sustain. Chem. Eng. 2017, 5, 2783–2798. [Google Scholar] [CrossRef]
- Liu, H.; Elkamel, A.; Lohi, A.; Biglari, M. Computational fluid dynamics modeling of biomass gasification in circulating fluidized-bed reactor using the Eulerian-Eulerian approach. Ind. Eng. Chem. Res. 2013, 52, 18162–18174. [Google Scholar] [CrossRef]
- Gerber, S.; Oevermann, M. A two dimensional Eulerian-Lagrangian model of wood gasification in a charcoal bed—Part I: Model description and base scenario. Fuel 2014, 115, 385–400. [Google Scholar] [CrossRef]
- Solar, J.; de Marco, I.; Caballero, B.M.; Lopez-Urionabarrenechea, A.; Rodriguez, N.; Agirre, I.; Adrados, A. Influence of temperature and residence time in the pyrolysis of woody biomass waste in a continuous screw reactor. Biomass Bioenergy 2016, 95, 416–423. [Google Scholar] [CrossRef]
- Hu, C.; Luo, K.; Wang, S.; Sun, L.; Fan, J. Computational fluid dynamics/discrete element method investigation on the biomass fast pyrolysis: The influences of shrinkage patterns and operating parameters. Ind. Eng. Chem. Res. 2019, 58, 1404–1416. [Google Scholar] [CrossRef]
- Mehrabian, R.; Scharler, R.; Obernberger, I. Effects of pyrolysis conditions on the heating rate in biomass particles and applicability of TGA kinetic parameters in particle thermal conversion modeling. Fuel 2012, 93, 567–575. [Google Scholar] [CrossRef]
- Zhou, T.; Yang, S.; Wei, Y.; Hu, J.; Wang, H. Impact of wide particle size distribution on the gasification performance of biomass in a bubbling fluidized bed gasifier. Renew. Energy 2020, 148, 534–547. [Google Scholar] [CrossRef]
- Goldschmidt, M.; Beetstra, R.; Kuipers, J. Hydrodynamic modelling of dense gas-fluidised beds: Comparison and validation of 3D discrete particle and continuum models. Powder Technol. 2004, 142, 23–47. [Google Scholar] [CrossRef]
- Van Sint Annaland, M.; Bokkers, G.A.; Goldschmidt, M.; Olaofe, O.O.; van der Hoef, M.A.; Kuipers, J. Development of a multi-fluid model for poly-disperse dense gas-solid fluidized-beds, part II: Segregation in binary particle mixtures. Chem. Eng. Sci. 2009, 64, 4237–4246. [Google Scholar] [CrossRef]
- Ku, X.; Li, T.; Løvås, T. Eulerian-Lagrangian simulation of biomass gasification behavior in a high-temperature entrained-flow reactor. Energy Fuels 2014, 28, 5184–5196. [Google Scholar] [CrossRef] [Green Version]
- Ku, X.; Li, T.; Løvås, T. CFD-DEM simulation of biomass gasification with steam in a fluidized-bed reactor. Chem. Eng. Sci. 2015, 122, 270–283. [Google Scholar] [CrossRef] [Green Version]
- Ku, X.; Jin, H.; Lin, J. Comparison of gasification performances between raw and torrefied biomasses in an air-blown fluidized-bed gasifier. Chem. Eng. Sci. 2017, 168, 235–249. [Google Scholar] [CrossRef]
- Backreedy, R.I.; Fletcher, L.M.; Ma, L.; Pourkashanian, M.; Williams, A. Modelling pulverised coal combustion using a detailed coal combustion model. Combust. Sci. Technol. 2006, 178, 763–787. [Google Scholar] [CrossRef]
- Gidaspow, D. Multiphase Flow and Fluidization; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Tsuji, Y.; Tanaka, T.; Ishida, T. Lagrangian numerical simulation of plug flow of cohesionless particles in a horizontal pipe. Powder Technol. 1992, 71, 239–250. [Google Scholar] [CrossRef]
- OpenCFD, Ltd. OpenFOAM—The Open Source CFD Toolbox—User Guide, Version 2.1.1; OpenCFD, Ltd.: Reading, UK, 2012. [Google Scholar]
- Kantarelis, E.; Yang, W.; Blasiak, W. Production of liquid feedstock from biomass via steam pyrolysis in a fluidised bed reactor. Energy Fuel 2013, 27, 4748–4759. [Google Scholar] [CrossRef]
- Mellin, P.; Kantarelis, E.; Yang, W. Computational fluid dynamics modeling of biomass fast pyrolysis in a fluidized-bed reactor, using a comprehensive chemistry scheme. Fuel 2014, 117, 704–715. [Google Scholar] [CrossRef]
- Pan, Y.; Kong, S. Predicting effects of operating conditions on biomass fast pyrolysis using particle-level simulation. Energy Fuels 2017, 31, 635–646. [Google Scholar] [CrossRef]
- Calonaci, M.; Grana, R.; Hemings, E.B.; Bozzano, G.; Dente, M.; Ranzi, E. Comprehensive kinetic modeling study of bio-oil formation from fast pyrolysis of biomass. Energy Fuels 2010, 24, 5727–5734. [Google Scholar] [CrossRef]
- Varhegyi, G.; Antal, M.J. Kinetics of the thermal decomposition of cellulose, hemicellulose, and sugar cane bagasse. Energy Fuels 1989, 3, 329–335. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Magdziarz, A.; Dziok, T.; Sieradzka, M.; Nowak, W. Laboratory studies on the influence of biomass particle size on pyrolysis and combustion using TG GC/MS. Fuel 2019, 252, 635–645. [Google Scholar] [CrossRef]
- Burton, A.; Wu, H. Influence of biomass particle size on bed agglomeration during biomass pyrolysis in fluidized bed. Proc. Combust. Inst. 2017, 36, 2199–2205. [Google Scholar] [CrossRef]
- Efika, C.E.; Onwudili, J.A.; Williams, P.T. Influence of heating rates on the products of high-temperature pyrolysis of waste wood pellets and biomass model compounds. Waste Manag. 2018, 76, 497–506. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
















| Mass: |
| Momentum: |
| Energy: |
| Drag correlation: |
| Parameter | Value | |
|---|---|---|
| Reactor | geometry (m) | 0.072 × 0.65 × 0.00085 |
| wall temperature (°C) | 500 | |
| inlet gas temperature (°C) | 463 | |
| inlet gas flow rate (g/s) | 0.05 | |
| Sand particle | shape | sphere |
| diameter (μm) | 850 | |
| density (kg/m3) | 2650 | |
| Biomass particle | shape | sphere |
| diameter (μm) | 450, 650, 850 | |
| material | mixture of pine and spruce | |
| density (kg/m3) | 600 | |
| feeding rate (g/s) | 0.00835 | |
| Resolution | grid | 20 × 130 × 1 |
| time step (s) | 1.0 × 10−5 |
| Biomass | Lignocellulose | ||
|---|---|---|---|
| moisture | 9.8 | cellulose | 44.0297 |
| ash | 0.3067 | hemicellulose | 21.9245 |
| lignocellulose | 89.8933 | LIG-H | 16.2854 |
| LIG-O | 6.4921 | ||
| LIG-C | 11.2683 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Ku, X.; Lin, J.; Ström, H. CFD-DEM Simulation of Biomass Pyrolysis in Fluidized-Bed Reactor with a Multistep Kinetic Scheme. Energies 2020, 13, 5358. https://doi.org/10.3390/en13205358
Chen T, Ku X, Lin J, Ström H. CFD-DEM Simulation of Biomass Pyrolysis in Fluidized-Bed Reactor with a Multistep Kinetic Scheme. Energies. 2020; 13(20):5358. https://doi.org/10.3390/en13205358
Chicago/Turabian StyleChen, Tao, Xiaoke Ku, Jianzhong Lin, and Henrik Ström. 2020. "CFD-DEM Simulation of Biomass Pyrolysis in Fluidized-Bed Reactor with a Multistep Kinetic Scheme" Energies 13, no. 20: 5358. https://doi.org/10.3390/en13205358