Reduction of Unburned Carbon Release and NOx Emission from a Pulverized Wood Pellet Boiler Retrofitted for Fuel Switching from Coal
Abstract
:1. Introduction
2. Target Boilers and Numerical Methods
2.1. Target Boiler and Operation Conditions
2.2. CFD Modeling Methods
3. Results and Discussion
3.1. Comparison of CFD Results with Measured Data for the Reference Case
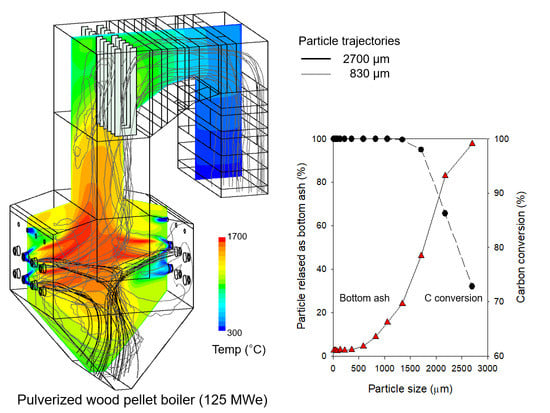
3.2. CFD Results for Flow and Reaction Characteristics in Case R
3.3. CFD Results for Influence of Air Staging (Cases 1–3)
3.4. CFD Results for Decrease in Particle Sizes (Cases 4–6)
3.5. CFD Results for Other Modifications in Boiler Operation (Cases 7 and 8)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Pre-exponential factor (s−1), surface area (m2) |
| c | Specific heat (J/kg·K) |
| CFD | Computational fluid dynamics |
| d | Diameter (cm) |
| E | Activation energy (kJ/kmol) |
| ECO | Economizer |
| FEGT | Furnace exit gas temperature (°C) |
| FGR | Flue gas recirculation |
| h | Convection coefficient (W/m2·K) |
| IRZ | Internal recirculation zone |
| Keq | Chemical equilibrium constant |
| k | Reaction rate (g·cm−2·atm−1·s−1) |
| Nu | Nusselt number |
| OFA | Overfire air |
| P | Pressure (atm) |
| Pr | Prandtl number |
| R | Universal gas constant, Reaction rate of char (g·cm−2·s−1) |
| Re | Reynolds number |
| SH | Superheater |
| SR | Stoichiometric ratio |
| T | Temperature (K) |
| t | Time (s) |
| UBC | Unburned carbon |
| UCSM | Unreacted core shrinking model |
| V | Volatile matter (kg) |
| v | Velocity (m/s) |
| Y | Unreacted char core to particle diameter ratio |
| θR | Radiation temperature |
| ε | Porosity of the ash layer, Emissivity |
| σ | Stefan–Boltzmann constant (5.67 × 10−8 (W/m2·K4)) |
| ax | axial direction |
| char | Unreacted char core |
| diff | Diffusion rate |
| dash | Diffusion rate in the ash layer |
| i | Index of char conversion reaction |
| o | Initial |
| p | Particle |
| s | Surface |
| t | Total pressure |
| tan | Tangential direction |
References
- Fernando, R. Fuels for Biomass Cofiring; CCC/102; IEA Clean Coal Centre: London, UK, 2005. [Google Scholar]
- Sloss, L.L. Emissions from Cofiring Coal, Biomass and Sewage Sludge; CCC/175; IEA Clean Coal Centre: London, UK, 2010. [Google Scholar]
- Van Loo, S.; Koppejan, J. The Handbook of Biomass Combustion and Cofiring; Earthscan: London, UK, 2008. [Google Scholar]
- Hupa, M.; Karlström, O.; Vainio, E. Biomass combustion technology development–It is all about chemical details. Proc. Combust. Inst. 2017, 36, 113–134. [Google Scholar] [CrossRef]
- Esteban, L.S.; Carrasco, J.E. Evaluation of different strategies for pulverization of forest biomasses. Powder Technol. 2006, 166, 139−151. [Google Scholar] [CrossRef]
- Saastamoinen, J.; Aho, M.; Moilanen, A.; Sørensen, L.H.; Clausen, S.; Berg, M. Burnout of pulverized biomass particles in large scale boiler–single particle model approach. Biomass Bioenergy 2010, 34, 728−736. [Google Scholar] [CrossRef]
- Panahi, A.; Tarakcioglu, M.; Schiemann, M.; Delichatsios, M.; Levendis, Y.A. On the particle sizing of torrefied biomass for co-firing with pulverized coal. Combust. Flame 2018, 194, 72−84. [Google Scholar] [CrossRef]
- Magalhães, D.; Panahi, A.; Kazanç, F.; Levendis, Y.A. Comparison of single particle combustion behaviours of raw and torrefied biomass with Turkish lignites. Fuel 2019, 241, 1085−1094. [Google Scholar] [CrossRef]
- Li, J.; Paul, M.C.; Younger, P.L.; Watson, I.; Hossain, M.; Welch, S. Characterization of biomass combustion at high temperatures based on an upgraded single particle model. Appl. Energy 2015, 156, 749−755. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Paul, M.C.; Younger, P.L.; Watson, I.; Hossain, M.; Welch, S. Prediction of high-temperature rapid combustion behaviour of woody biomass particles. Fuel 2016, 165, 205−214. [Google Scholar] [CrossRef] [Green Version]
- Mason, P.E.; Darvell, L.I.; Jones, J.M.; Pourkashanian, M.; Williams, A. Single particle flame-combustion studies on solid biomass fuels. Fuel 2015, 151, 21−30. [Google Scholar] [CrossRef]
- Karlström, O.; Hupa, L. Energy conversion of biomass char: Oxidation rates in mixtures of O2/CO2/H2O. Energy 2019, 181, 615−624. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1−15. [Google Scholar] [CrossRef]
- Williams, A.; Jones, J.M.; Pourkashanian, M. Pollutants from the combustion of solid biomass fuels. Prog. Energy Combust. Sci. 2012, 38, 113−137. [Google Scholar] [CrossRef]
- Nalbandian, H. NOx Control for Coal-Fired Plant; CCC/157; IEA Clean Coal Centre: London, UK, 2009. [Google Scholar]
- Glarborg, P.; Jensen, A.D.; Johnsson, J.E. Fuel nitrogen conversion in solid fuel fired systems. Prog. Energy Combust. Sci. 2003, 29, 89−113. [Google Scholar] [CrossRef]
- Riaza, J.; Mason, P.; Jones, J.M.; Gibbins, J.; Chalmers, H. High temperature volatile yield and nitrogen partitioning during pyrolysis of coal and biomass fuels. Fuel 2019, 248, 215–220. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Z.; Deng, S.; Xiong, Y.; Tan, H. Effect of biomass/coal co-firing and air staging on NOx emission and combustion efficiency in a drop tube furnace. Energy Procedia 2014, 61, 2331−2334. [Google Scholar] [CrossRef] [Green Version]
- Jo, H.; Kang, K.; Park, J.; Ryu, C.; Ahn, H.; Go, Y. Optimization of air distribution to reduce NOx emission and unburned carbon for the retrofit of a 500 MWe tangential-firing coal boiler. Energies 2019, 12, 3281. [Google Scholar] [CrossRef] [Green Version]
- Jo, H.; Kang, K.; Park, J.; Ryu, C.; Ahn, H.; Go, Y. Detailed assessment of mesh sensitivity for CFD simulation of coal combustion in a tangential-firing boiler. J. Mech. Sci. Technol. 2020, 34, 917–930. [Google Scholar] [CrossRef]
- Haider, A.; Levenspiel, O. Drag coefficient and terminal velocity of spherical and nonspherical particles. Powder Technol. 1989, 58, 63–70. [Google Scholar] [CrossRef]
- Li, J.; Brzdekiewicz, A.; Yang, W.; Blasiak, W. Co-firing based on biomass torrefaction in a pulverized coal boiler with aim of 100% fuel switching. Appl. Energy 2012, 99, 344–354. [Google Scholar]
- Ranz, W.E.; Marshall, W.R. Evaporation from Drops. Part I. Chem. Eng. Prog. 1952, 48, 141–146. [Google Scholar]
- Niksa, S. PC Coal Lab Version 4.1: User Guide and Tutorial; Niksa Energy Associates LLC.: Belmont, MA, USA, 1997. [Google Scholar]
- Wen, C.Y.; Chaung, T.Z. Entrainment coal Gasification Modeling. Ind. Eng. Chem. Process. Des. Dev. 1979, 18, 684–695. [Google Scholar] [CrossRef]
- Smith, K.L.; Smoot, L.D.; Fletcher, T.H.; Pugmire, R.J. The Structure and Reaction Processes of Coal; Plenum Press: New York, NY, USA, 1994. [Google Scholar]
- Jones, W.P.; Lindstedt, R.P. Global reaction schemes for hydrocarbon combustion. Combust. Flame 1988, 73, 233–249. [Google Scholar] [CrossRef]
- Magnussen, B.F.; Hjertager, B.H. On mathematical models of turbulent combustion with special emphasis on soot formation and combustion. Proc. Combust. Inst. 1977, 16, 719–729. [Google Scholar]
- Shih, T.H.; Liou, W.W.; Shabbir, A.; Yang, Z.; Zhu, J. A new k-ε eddy-viscosity model for high Reynolds number turbulent flows - model development and validation. Comput. Fluids 1995, 24, 227–238. [Google Scholar]
- Smith, T.F.; Shen, Z.F.; Friedman, J.N. Evaluation of coefficients for the weighted sum of gray gases model. J. Heat Transf. 1982, 104, 602–608. [Google Scholar] [CrossRef]
- Park, J.K.; Park, S.; Kim, M.K.; Ryu, C.; Baek, S.H.; Kim, Y.J.; Kim, H.H.; Park, H.Y. CFD analysis of combustion characteristics for fuel switching to bioliquid in oil-fired power plant. Fuel 2015, 159, 324–333. [Google Scholar] [CrossRef]
- Jakob, M. Heat transfer and flow resistance in cross flow of gases over tube banks. Trans. ASME 1938, 60, 384–386. [Google Scholar]
- Zukauskas, A. Heat transfer from tubes in crossflow. Adv. Heat Transf. 1972, 8, 93–160. [Google Scholar] [CrossRef]
- De Soete, G.G. Overall reaction rates of NO and N2 formation from fuel nitrogen. Proc. Combust. Inst. 1975, 15, 1093–1102. [Google Scholar] [CrossRef]
- Williams, O.; Newbolt, G.; Eastwick, C.; Kingman, S.; Giddings, D.; Lormor, S.; Lester, E. Influence of mill type on densified biomass comminution. Appl. Energy 2016, 182, 219−231. [Google Scholar] [CrossRef]
- Yu, S.; Park, J.; Kim, M.; Kim, H.; Ryu, C.; Lee, Y.; Yang, W.; Jeong, Y. Improving energy density and grindability of wood pellets by dry torrefaction. Energy Fuels 2019, 33, 8632–8639. [Google Scholar] [CrossRef]











| Parameter | Values |
|---|---|
| Wood pellet | Proximate analysis (% wet): Total moisture 8.90, volatile matter 73.77, fixed carbon 14.85, ash 2.48 Ultimate analysis (% dry, ash-free): C 49.65, H 5.62, O 44.32, N 0.41 Higher heating value (MJ/kg): 17.11 |
| Fuel throughput | 76,800 kg/h |
| Burner primary air | 122,920 kg/h, 159 °C at the mill inlet |
| Burner secondary air | 331,888 kg/h, 315 °C |
| OFA | 22,275 kg/h, 315 °C |
| Excess air ratio | 21.6% |
| Case | Burner Secondary Air Ratio (%) | Burner Zone Stoichiometric Ratio | OFA Ratio (%) | Note | ||
|---|---|---|---|---|---|---|
| F1 | F2 | R2 | ||||
| R | 23.0 | 23.0 | 23.0 | 1.16 | 4.7 | Reference case |
| 1 | 24.6 | 24.6 | 24.6 | 1.22 | 0 | Different air staging |
| 2 | 21.5 | 21.5 | 21.5 | 1.10 | 9.4 | |
| 3 | 18.4 | 18.4 | 18.4 | 0.99 | 18.7 | |
| 4 | 23.0 | 23.0 | 23.0 | 1.16 | 4.7 | Smaller fuel particles (Figure 2) with different air staging |
| 5 | 21.5 | 21.5 | 21.5 | 1.10 | 9.4 | |
| 6 | 18.4 | 18.4 | 18.4 | 0.99 | 18.7 | |
| 7 | 23.0 | 23.0 | 23.0 | 1.16 | 4.7 | Lower swirl intensity (vtan/vax = 0.7) |
| 8 | 23.0 | 23.0 | 23.0 | 1.16 | 4.7 | Flue gas recirculation (20%) |
| Category | Submodels |
|---|---|
| Discrete phase | —Lagrangian scheme with stochastic tracking for turbulence —Number of particles: 74,800 —Particle size: 10–2700 μm |
| Biomass combustion | —Devolatilization: bio-FLASHCHAIN [24] Dry biomass→77.80 wt.% daf volatiles + 19.62 wt.% daf C(s) (Char) Composition of volatiles: Tar 31.2, CO 34.1, CO2 5.7, H2O 11.6, H2 0.91, CH4 1.19, CxHy 8.31 wt.% daf Devolatilization rate: ; E = 18.5 kcal/mol, A= 1.03 × 107 s−1 —Char conversion: unreacted core shrinking model [25] , (R1) C(s) + 0.5 O2 → CO (R2) C(s) + H2O → CO+H2 (R3) C(s) + CO2 → 2 CO |
| Species, gas reaction | —Species: Tar, CO, CO2, H2, CH4, CxHy, H2, SO2, O2, N2 —Reaction mechanism [26,27] (R4) CxHyOz (tar) + a x2+y4-z2)O2 → x CO + 0.5y H2 (R5) CnHm + 0.5n x2+y4-z2)O2 → n CO + 0.5m H2 (R6) CnHm + 0.5n Hx2+y4-z2)2O → n CO + 0.5(m+n) H2 (R7) CH4 + 0.5 x2+y4-z2)O2 → CO + 2 H2 (R8) CH4 + 0.5 Hx2+y4-z2)2O → CO + 2.5 H2 (R9) CO + H2O → CO2 + H2 (R10) H2 + 0.5 O2 → H2O —Reaction rate: kinetic rate/eddy dissipation rate model [28] |
| NOx | —Thermal NOx: extended Zeldovich mechanism —Fuel NOx: De Soete model [34] —Fuel-N evenly distributed between volatile-N as NH3 and char-N as NO —NO reduction on the particle surface with a N2-BET surface area of 200 m2/g |
| Parameter | Measured Data | CFD | |
|---|---|---|---|
| Exit O2 (% dry) | 3.72 | 4.1 | |
| Exit gas temperature (°C) | 354.5 | 367.6 | |
| Exit NO (ppm, 6% O2) | 81.2 | 107.6 | |
| Heat absorption (MWth) | Evaporator | 148.4 | 148.8 |
| Platen+ Primary SH | 65.1 | 65.4 | |
| Final SH | 31.6 | 31.9 | |
| RH | 46.0 | 46.5 | |
| Economizer | 15.7 | 15.0 | |
| UBC (wt%) | Bottom ash | 61.7 | 69.6 |
| Fly ash | 1.9 | 1.3 | |
| Case | Burner Zone Stoichiometric Ratio | Unburned Carbon Content (%) | Bottom Ash Release (kg/h) | Carbon Conversion (%) | Boiler Efficiency (%) | Furnace Exit Gas Temp. (°C) | Exit NO (ppm, 6% O2) | |
|---|---|---|---|---|---|---|---|---|
| Fly Ash | Bottom Ash | |||||||
| R | 1.16 | 1.3 | 69.6 | 990.0 | 97.84 | 84.3 | 1117.8 | 107.6 |
| 1 | 1.22 | 2.8 | 68.7 | 984.9 | 97.81 | 84.2 | 1140.9 | 120.1 |
| 2 | 1.10 | 1.6 | 70.7 | 1066.6 | 97.63 | 84.0 | 1115.4 | 98.5 |
| 3 | 0.99 | 2.4 | 76.6 | 1663.9 | 96.02 | 82.8 | 1099.4 | 84.3 |
| 4 | 1.16 | 0.7 | 3.5 | 143.4 | 99.95 | 86.0 | 1138.9 | 111.1 |
| 5 | 1.10 | 1.6 | 5.9 | 139.3 | 99.89 | 85.9 | 1135.6 | 101.3 |
| 6 | 0.99 | 3.5 | 31.6 | 287.4 | 99.54 | 85.6 | 1125.9 | 85.4 |
| 7 | 1.16 | 0.7 | 71.4 | 1079.9 | 97.63 | 84.0 | 1112.0 | 108.4 |
| 8 | 1.16 | 6.2 | 69.3 | 906.8 | 97.76 | 82.8 | 1086.9 | 179.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Yu, S.; Park, J.; Jo, H.; Park, J.; Ryu, C.; Jeong, Y.-g. Reduction of Unburned Carbon Release and NOx Emission from a Pulverized Wood Pellet Boiler Retrofitted for Fuel Switching from Coal. Energies 2020, 13, 5077. https://doi.org/10.3390/en13195077
Lee J, Yu S, Park J, Jo H, Park J, Ryu C, Jeong Y-g. Reduction of Unburned Carbon Release and NOx Emission from a Pulverized Wood Pellet Boiler Retrofitted for Fuel Switching from Coal. Energies. 2020; 13(19):5077. https://doi.org/10.3390/en13195077
Chicago/Turabian StyleLee, Jiseok, Seunghan Yu, Jinje Park, Hyunbin Jo, Jongkeun Park, Changkook Ryu, and Yeong-gap Jeong. 2020. "Reduction of Unburned Carbon Release and NOx Emission from a Pulverized Wood Pellet Boiler Retrofitted for Fuel Switching from Coal" Energies 13, no. 19: 5077. https://doi.org/10.3390/en13195077