Techno-Economic Analysis of ZnO Nanoparticles Pretreatments for Biogas Production from Barley Straw
Abstract
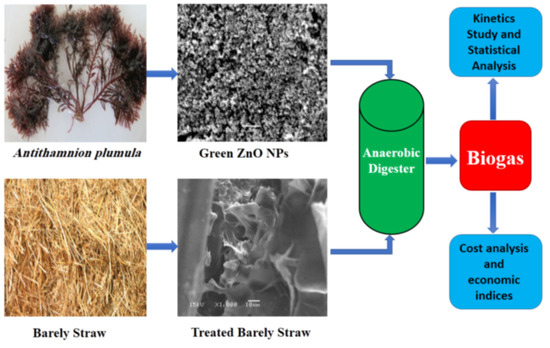
:1. Introduction
2. Materials and Methods
2.1. Inoculum and Substrates Preparation
2.2. Biogas Experiment
2.3. Preparation of Extract from A. plumula
2.4. Synthesis of ZnO Nanoparticles (ZnO NPs)
2.5. Characterization and Measurement
2.6. Kinetics Study and Statistical Analysis
2.7. Cost Analysis and Economic Indices
- The initial cost of investment expenditures in year t (I)
- Fuel expenditures (if applicable) in year t (F)
- The sum of all electricity generated in year t (E)
- The discount rate of the project (r)
- The life of the system (n)
3. Results and Discussion
3.1. GC-MS Analysis
3.2. Characterization for Barley Straw
3.2.1. Fourier Transform Infrared Spectra (FTIR)
3.2.2. X-ray Diffractometry (XRD)
3.2.3. Scanning Electron Microscopy (SEM)
3.2.4. Thermogravimetric Analysis (TGA)
3.3. ZnO NPs Characterization
3.3.1. Fourier Transform Infrared Spectra (FTIR)
3.3.2. X-ray Diffractograms
3.3.3. SEM Microscopy and EDX
3.3.4. Transmission Electron Microscope (TEM)
3.3.5. BET Analysis Surface Area
3.4. Chemical Compositions of Barley Straw
3.5. Effect of Both Mechanical Pretreatment and ZnO NPs on Biogas Production
3.6. The Proposed Mechanism of ZnO NPs in Biogas Production
3.7. Kinetic Study
3.8. The Cost-Benefit Analysis
3.9. Levelized Cost of Energy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Anaerobic digestion |
| NPs | Nanoparticles |
| ZnO NPs | Zinc oxide nanoparticles |
| LCOE | Levelized cost of energy |
| NPV | Net present value |
| GC/MS | Gas chromatography mass spectrometry |
| FTIR | Fourier transform infrared |
| FW | Full width |
| XRD | X-ray diffractograms |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscope |
| EDX | Energy-dispersive X-ray spectroscopy |
| BET | Brunauer–Emmett–Teller |
| TGA | Thermogravimetric analysis |
| TS | Total solids |
| Rm | The maximum biogas production rate (L/g VS added) |
| VS | Volatile solids |
| λ | The lag phase time (days) |
References
- Carlini, M.; Mosconi, E.M.; Castellucci, S.; Villarini, M.; Colantoni, A. An economical evaluation of anaerobic digestion plants fed with organic agro-industrial waste. Energies 2017, 10, 1165. [Google Scholar] [CrossRef]
- Council of the European Union. Directive of the European Parliament and of the Council on the Promotion of the Use of Energy from Renewable Sources; Council of the European Union: Brussels, Belgium, 2018. [Google Scholar]
- Tsavkelova, E.A.; Netrusov, A.I. Biogas production from cellulose containing substrates: A review. Appl. Biochem. Microbiol. 2012, 48, 421–433. [Google Scholar] [CrossRef]
- Appels, L.; Lauwers, J.; Degrève, J.; Helsen, L.; Lievens, B.; Willems, K.; van Impe, J.; Dewil, R. Anaerobic digestion in global bio-energy production: Potential and research challenges. Renew. Sustain. Energy Rev. 2011, 15, 4295–4301. [Google Scholar] [CrossRef]
- Feng, L.; Perschke, Y.M.L.; Fontaine, D.; Ward, A.J.; Eriksen, J.; Sorensen, P.; Moller, H.B. Co-ensiling of cover crops and barley straw for biogas production. Renew. Energy 2019, 142, 677–683. [Google Scholar] [CrossRef]
- Monlau, F.; Barakat, A.; Trably, E.; Dumas, C.; Steyer, J.P.; Carrere, C. Lignocellulosic materials into biohydrogen and biomethane: Impact of structural features and pretreatment. Crit. Rev. Environ. Sci. Technol. 2011, 43, 260–322. [Google Scholar] [CrossRef]
- Guerrieri, A.S.; Anifantis, A.S.; Santoro, F.; Pascuzzi, S. Study of a Large Square Baler with Innovative Technological Systems that Optimize the Baling Effectiveness. Agriculture 2019, 9, 86. [Google Scholar] [CrossRef] [Green Version]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Müller, B.; Schnürer, A. Biogas production from wheat straw: Community structure of cellulose-degrading bacteria. Energy Sustain. Soc. 2013, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Antognoni, S.; Ragazzi, M.; Rada, E.C.; Plank, R.; Aichinger, P.; Kuprian, M.; Ebner, C. Potential Effects of Mechanical Pre-treatments on Methane Yield from Solid Waste Anaerobically Digested. Int. J. Environ. Bioremed. Biodegrad. 2013, 1, 20–25. [Google Scholar]
- Jędrczak, A.; Królik, D. Influence of paper particle size on the efficiency of digestion process. Environ. Prot. Eng. 2007, 33, 145–155. [Google Scholar]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2015, 199, 386–397. [Google Scholar] [CrossRef]
- Kratky, L.; Jirout, T. Biomass size reduction machines for enhancing biogas production. Chem. Eng. Technol. 2011, 34, 391–399. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Angelidaki, I. Biogas production from ensiled meadow grass; effect of mechanical pretreatments and rapid determination of substrate biodegradability via physicochemical methods. Bioresour. Technol. 2015, 182, 329–335. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Egelund, H.; Larsen, U.; Pedersen, J.; Trenel, P.; Angelidaki, I. Mechanical pretreatment at harvesting increases the bioenergy output from marginal land grasses. Renew. Energy 2017, 111, 914–921. [Google Scholar] [CrossRef] [Green Version]
- Balsari, P.; Menardo, S.; Airoldi, G. Effect of physical and thermal pre-treatment on biogas yield of some agricultural by-products. In Progress in Biogas II; German Society for Sustainable Biogas and Bioenergy Utilisation: Stuttgart, Hohenheim, Germany, 2011. [Google Scholar]
- Abdelsalam, E.; Samer, M.; Attia, Y.A.; AbdelHadi, M.A.; Hassan, H.E.; Badr, Y. Influence of zero valent iron nanoparticles and magnetic iron oxide nanoparticles on biogas and methane production from anaerobic digestion of manure. Energy J. 2017, 120, 842–853. [Google Scholar] [CrossRef]
- Amirante, R.; Demastro, G.; Distaso, E.; Hassaan, M.A.; Mormando, A.; Pantaleo, A.M.; Tamburrano, P.; Tedone, L.; Clodoveo, M.L. Effects of ultrasound and green synthesis ZnO nanoparticles on biogas production from Olive Pomace. Energy Proced. 2018, 148, 940–947. [Google Scholar]
- Mu, H.; Chen, Y.; Xiao, N. Effects of metal oxide nanoparticles (TiO2, Al2O3, SiO2 and ZnO) on waste activated sludge anaerobic digestion. Bioresour. Technol. 2011, 102, 10305–10311. [Google Scholar] [CrossRef]
- Mu, H.; Chen, Y. Long-term effect of ZnO nanoparticles on waste activated sludge anaerobic digestion. Water Res. 2011, 45, 5612–5620. [Google Scholar] [CrossRef]
- Hassaan, M.A.; Pantaleo, A.; Tedone, L.; Elkatory, M.R.; Ali, R.M.; Nemr, A.E.; Mastro, G.D. Enhancement of biogas production via green ZnO nanoparticles: Experimental results of selected herbaceous crops. Chem. Eng. Commun. 2019, 1–14. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Devi Rajeswari, V. A review on green synthesis, biomedical applications, and toxicity studies of ZnO NPs. Bioinorganic Chem. Appl. 2018, 2018, 3569758. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, K.; Zheng, L.; Han, X.; Xu, Y.; Li, Y.; Li, S.; Xu, X.; Zhang, H.; Zhao, L. Modelling Biogas Production Kinetics of Various Heavy Metals Exposed Anaerobic Fermentation Process Using Sigmoidal Growth Functions. Waste Biomass Valoriz. 2020, 11, 4837–4848. [Google Scholar] [CrossRef]
- Pantaleo, A.; Villarini, M.; Colantoni, A.; Carlini, M.; Santoro, F.; RajabiHamedani, S. Techno-Economic Modeling of Biomass Pellet Routes: Feasibility in Italy. Energies 2020, 13, 1636. [Google Scholar] [CrossRef] [Green Version]
- RajabiHamedani, S.; Villarini, M.; Colantoni, A.; Carlini, M.; Cecchini, M.; Santoro, F.; Pantaleo, A. Environmental and Economic Analysis of an Anaerobic Co-Digestion Power Plant Integrated with a Compost Plant. Energies 2020, 13, 2724. [Google Scholar] [CrossRef]
- Said, M.; El-Shimy, M.; Abdelraheem, M.A. Photovoltaics energy: Improved modeling and analysis of the levelized cost of energy (LCOE) and grid parity–Egypt case study. Sustain. Energy Technol. Assess. 2015, 9, 37–48. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Waste Water; American Public Health Association: Washington, DC, USA, 1998; p. 874. [Google Scholar]
- Gelegenis, J.; Georgakakis, D.; Angelidaki, I.; Mavris, V. Optimization of biogas production by co-digesting whey with diluted poultry manure. Renew. Energ. 2007, 32, 2147–2160. [Google Scholar] [CrossRef]
- Trine, L.H.; Jens, E.S.; Irini, A.; Emilia, M.C.J.; Hans, M.; Thomas, H.C. Method for determination of methane potentials of solid organic waste. Waste Manag. 2004, 24, 393–400. [Google Scholar]
- Nielfa, A.; Cano, R.; FdzPolanco, M. Theoretical methane production generated by the co-digestion of organic fraction municipal solid waste and biological sludge. Biotechnol. Rep. 2015, 1, 14–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remigi, E.U.; Buckley, C.A. Co-Digestion of High Strength/Toxic Organic Effluents in Anaerobic Digesters at Wastewater Treatment Works; Water Research Commission: Pretoria, South Africa, 2016. [Google Scholar]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process. 2015, 32, 55–61. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; ElAzab, W.I.M.; Ali, H.R.; Mansour, M.S.M. Green synthesis and characterization of ZnO nanoparticles for photocatalytic degradation of anthracene. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Jeon, B.H.; Jeung, J.H.; Rene, E.R.; Banu, J.R.; Ravindran, B.; Vu, C.M.; Ngo, H.H.; Guo, W.; Chang, S.W. Thermophilic Anaerobic Digestion of Model Organic Wastes: Evaluation of Biomethane Production and Multiple Kinetic Models Analysis. Bioresour. Technol. 2019, 280, 269–276. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L. Comparison on Batch Anaerobic Digestion of Five Different Livestock Manures and Prediction of Biochemical Methane Potential (BMP) Using Different Statistical Models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef] [Green Version]
- Donoso-Bravo, A.; Pérez-Elvira, S.I.; Fdz-Polanco, F. Application of Simplified Models for Anaerobic Biodegradability Tests. Evaluation of Pre-Treatment Processes. Chem. Eng. J. 2010, 160, 607–614. [Google Scholar] [CrossRef]
- Li, L.; He, Q.; Zhao, X.; Wu, D.; Wang, X.; Peng, X. Anaerobic Digestion of Food Waste: Correlation of Kinetic Parameters with Operational Conditions and Process Performance. Biochem. Eng. J. 2018, 130, 1–9. [Google Scholar] [CrossRef]
- Mohammed, M.; Egyir, I.S.; Donkor, A.K.; Amoah, P.; Nyarko, S.; Boateng, K.K.; Ziwu, C. Feasibility study for biogas integration into waste treatment plants in Ghana. Egypt. J. Pet. 2017, 26, 695–703. [Google Scholar] [CrossRef] [Green Version]
- Uellendahl, H.; Wang, G.; Møller, H.B.; Jørgensen, U.; Skiadas, I.V.; Gavala, H.N.; Ahring, B.K. Energy balance and cost-benefit analysis of biogas production from perennial energy crops pretreated by wet oxidation. Water Sci. Technol. 2008, 58, 1841–1847. [Google Scholar] [CrossRef] [Green Version]
- Schneider, L.; Haverinen, J.; Jaakkola, M.; Lassi, U. Pretreatment and fractionation of lignocellulosic barley straw by mechanocatalysis. Chem. Eng. J. 2017, 327, 898–905. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Wang, Q.; Jianga, Z.H.; Yang, X.; Ji, Y. Enzymatic hydrolysis of pretreated soybean straw. Biomass Bioenergy 2007, 31, 162–167. [Google Scholar] [CrossRef]
- Karuppiah, T.; Azariah, V.E. Biomass pretreatment for enhancement of biogas production. In Anaerobic Digestion; IntechOpen: London, UK, 2019. [Google Scholar]
- Sanchez-Silva, L.; López-González, D.; Villaseñor, J.; Sánchez, P.; Valverde, J.L. Thermogravimetric-mass spectrometric analysis of lignocellulosic and marine biomass pyrolysis. Bioresour. Technol. 2012, 109, 163–172. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Hassaan, M.; El Katory, M.; Ali, R.M.; El Nemr, A. Photocatalytic degradation of reactive black 5 using Photo-Fenton and ZnO nanoparticles under UV irradiation. Egypt. J. Chem. 2020, 63, 17–18. [Google Scholar] [CrossRef]
- Soliman, E.A.; Elkatory, M.R.; Hashem, A.I.; Ibrahim, H.S. Synthesis and performance of maleic anhydride copolymers with alkyl linoleate or tetra-esters as pour point depressants for waxy crude oil. Fuel 2018, 211, 535–547. [Google Scholar] [CrossRef]
- Ali, R.M.; Elkatory, M.R.; Hamad, H.A. Highly active and stable magnetically recyclable CuFe2O4 as a heterogenous catalyst for efficient conversion of waste frying oil to biodiesel. Fuel 2020, 268, 117297. [Google Scholar] [CrossRef]
- Xu, C.X.; Sun, X.W.; Dong, Z.L.; Cui, Y.P.; Wang, B.P. Nanostructured singlecrystalline twin disks of zinc oxide. Cryst. Growth Des. 2007, 7, 541–544. [Google Scholar] [CrossRef]
- Ashkenov, N.; Mbenkum, B.N.; Bundesmann, C.; Riede, V.; Lorenz, M.; Spemann, D.; Kaidashev, M.; Kasic, A.; Schubert, M.; Grundmann, M.; et al. Infrared dielectric functions and phonon modesof high-quality ZnO films. J. Appl. Phys. 2003, 93, 1. [Google Scholar] [CrossRef]
- Alim, K.A.; Fonoberov, V.A.; Balandin, A.A. Origin of the optical phonon frequency shifts in ZnO quantum dots. Appl. Phys. Lett. 2005, 86, 05103. [Google Scholar] [CrossRef]
- Sivakami, R.; Dhanuskodi, S.; Karvembu, R. Estimation of lattice strain in nanocrystalline RuO2 by Williamson–Hall and size–strain plot methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 43. [Google Scholar] [CrossRef]
- Ali, R.M.; Hassaan, M.A.; Elkatory, M.R. Towards Potential Removal of Malachite Green from Wastewater: Adsorption Process Optimization and Prediction. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Suisse, 2020; p. 1008. [Google Scholar]
- Ismail, M.A.; Taha, K.K.; Modwi, A.; Khezami, L. ZnO nanoparticles: Surface and X-ray profile analysis. J. Ovonic Res. 2018, 14, 381–393. [Google Scholar]
- Khan, M.; Cao, W. Cationic (V, Y)-codoped TiO2 with enhanced visible light induced photocatalytic activity: A combined experimental and theoretical study. J. Appl. Phys. 2013, 114, 183514. [Google Scholar] [CrossRef]
- Burton, A.W.; Ong, K.; Rea, T.; Chan, I.Y. On the estimation of average crystallite size of zeolites from the Scherrer equation: A critical evaluation of its application to zeolites with one-dimensional pore systems. Microporous Mesoporous Mater. 2009, 117, 75–90. [Google Scholar] [CrossRef]
- Friis, J.; Holm, C.; Halling-Sørensen, B. Evaluation of elemental composition of algal biomass as toxical endpoint. Chemosphere 1998, 13, 2665–2676. [Google Scholar] [CrossRef]
- Pang, Y.Z.; Liu, Y.P.; Li, X.J.; Wang, K.S.; Yuan, H.R. Improving biodegradability and biogas production of corn stover through sodium hydroxide solid state pretreatment. Energy Fuels 2008, 22, 2761–2766. [Google Scholar] [CrossRef]
- Nyns, E.J. Biomethanation Processes; Wiley-VCH: Weinheim, Berlin, Germany, 1986; pp. 207–267. [Google Scholar]
- Kivaisi, A.K.; Mtila, M. Production of biogas from water hyacinth (Eichhorniacrassipes) in a two stage bioreactor. World J. Microbiol. Biotechnol. 1997, 14, 125–131. [Google Scholar] [CrossRef]
- Mshandete, A.; Kivaisi, A.; Rubindamayugi, M.; Mattiasson, B. Anaerobic batch co-digestion of sisal pulp and fish wastes. Bioresour. Technol. 2004, 95, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.A.; Pantaleo, A.; Tedone, L.; Demastro, G. Biogas production from silage flour wheat influenced by chemical and green synthesized ZnO nanoparticles. In Proceedings of the XLVII Conference of Italian Society for Agronomy, Marsala, Italy, 12–14 September 2018. [Google Scholar]
- Elijah, T.; Ibifuro, A.; Yahaya, S.M. The study of cow dung as co-substrate with rice husk in biogas production. Sci. Res. Essay 2009, 9, 861–866. [Google Scholar]
- Wang, T.; Zhang, D.; Dai, L.; Chen, Y.; Dai, X. Effects of metal nanoparticles on methane production from waste-activated sludge and microorganism community shift in anaerobic granular sludge. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Rahman, A.; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Zhang, Y.; Xiang, Y.; Xu, R.; Jia, M.; Cao, J.; Xiong, W. Effects of different conductive nanomaterials on anaerobic digestion process and microbial community of sludge. Bioresour. Technol. 2020, 304, 123016. [Google Scholar] [CrossRef]
- Ma, T.F.; Chen, Y.P.; Fang, F.; Yan, P.; Shen, Y.; Kang, J.; Nie, Y.D. Effects of ZnO nanoparticles on aerobic denitrifying bacteria Enterobacter cloacae strain HNR. Sci. Total Environ. 2020, 725, 138284. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Olaya, W.B. Effect of Size, Coating and Concentration of Zinc Oxide Nanoparticles on Anaerobic Digestion of Municipal Wastewater Sludge. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2019. [Google Scholar]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef]
- Ganzoury, M.A.; Allam, N.K. Impact of nanotechnology on biogas production: A mini-review. Renew. Sustain. Energy Rev. 2015, 50, 1392–1404. [Google Scholar] [CrossRef]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef] [PubMed]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Experimental and Kinetic Study on Anaerobic Digestion of FoodWaste: The E_ect of Total Solids and PH. J. Renew. Sustain. Energy 2015, 7, 063104. [Google Scholar] [CrossRef]
- Mao, C.; Wang, X.; Xi, J.; Feng, Y.; Ren, G. Linkage of Kinetic Parameters with Process Parameters and Operational Conditions during Anaerobic Digestion. Energy 2017, 135, 352–360. [Google Scholar] [CrossRef]
- Abdelhady, S.; Borello, D.; Shaban, A. Assessment of levelized cost of electricity of offshore wind energy in Egypt. Wind Eng. 2017, 41, 160–173. [Google Scholar] [CrossRef]
- DLR. German Aerospace Center Concentrating Solar Power for the Mediterranean Region (Report by order of Federal Ministry for the Environment); DLR: Berlin, Germany, 2005; Available online: www.dlr.de/tt/med-csp (accessed on 30 July 2020).
- Hansen, K. Decision-making based on energy costs: Comparing levelized cost of energy and energy system costs. Energy Strategy Rev. 2019, 24, 68–82. [Google Scholar] [CrossRef]


















| Parameters | Cost |
|---|---|
| Initial Investment Cost (€) | 1000 |
| Annual Operations and Maintenance (O&M) Costs (€) | 100 |
| O&M Growth Rate (%) | 2% |
| Annual Fuel Costs (€) | - |
| Project Lifespan (years) | 10 |
| Discount Rate (%) | 8% |
| Position | Crystal Size (nm) Chemical ZnO NPs | Crystal Size (nm) Green ZnO NPs |
|---|---|---|
| 31.94° | 3.5 | 3.3 |
| 34.54° | 3.7 | 3.5 |
| 37.08° | 3.4 | 3.2 |
| 47.76° | 3.2 | 3.0 |
| 56.78° | 3.1 | 2.9 |
| 63.02° | 2.7 | 2.5 |
| 66.58° | 3.3 | 3.1 |
| 69.14° | 2.6 | 2.4 |
| 69.36° | 2.7 | 2.6 |
| 77.04° | 3.4 | 3.2 |
| Elements | Chemical | Green |
|---|---|---|
| Zn | 85.01 | 89.4 |
| O | 5.72 | 5.18 |
| Na | 9.27 | 5.42 |
| Total | 100% | 100% |
| Sample | BET Surface Area (m2/g) | Mean Pore Diameter (nm) | Total Pore Volume (cm3/g( |
|---|---|---|---|
| Chemical ZnO NPs | 24.33 | 10.370 | 0.0631 |
| Green ZnO NPs | 46.47 | 12.728 | 0.1479 |
| Component | Barley Straw | Inoculum |
|---|---|---|
| TS% | 51.9 | 8.40 |
| Ash% | 5.05 | 28.30 |
| VS% | 94.05 | 71.70 |
| N | 1.56 | 4.30 |
| C | 51.15 | 49.90 |
| H | 6.42 | 5.50 |
| C:N | 33.04 | 9.07 |
| First-Order Kinetic Model | |||||
|---|---|---|---|---|---|
| Chemical | |||||
| Samples | R2 | PredictedP(mL/gVS) | Differences% | K(1/day) | RMSE |
| (4 mm) | 0.995 | 282.03 | 1.1 | 0.387 | 5.5 |
| (0.4 mm) | 0.992 | 343.78 | 1.1 | 0.399 | 8.3 |
| 5 mg/L | 0.995 | 373.50 | 0.1 | 0.356 | 7.2 |
| 10 mg/L | 0.997 | 379.37 | 1.5 | 0.347 | 5.4 |
| 20 mg/L | 0.977 | 172.08 | 0.6 | 0.44 | 6.9 |
| Green | |||||
| 5 mg/L | 0.993 | 380.93 | 1.6 | 0.362 | 8.3 |
| 10 mg/L | 0.999 | 386.53 | 1.1 | 0.334 | 3.9 |
| 20 mg/L | 0.967 | 166.72 | 3.6 | 0.537 | 7.6 |
| Cone Model | |||||
| Chemical | |||||
| Samples | R2 | PredictedP(mL/gVS) | Differences% | K(1/day) | RMSE |
| (4 mm) | 0.760 | 275.96 | 1.1 | 0.329 | 21.62 |
| (0.4 mm) | 0.754 | 336.15 | 1.1 | 0.344 | 27.3 |
| 5 mg/L | 0.758 | 363.90 | 2.4 | 0.316 | 30.83 |
| 10 mg/L | 0.764 | 370.41 | 3.8 | 0.299 | 29.81 |
| 20 mg/L | 0.745 | 168.01 | 1.7 | 0.4 | 13.86 |
| Green | |||||
| 5 mg/L | 0.757 | 371.05 | 1.1 | 0.324 | 31.4 |
| 10 mg/L | 0.769 | 377.73 | 12.6 | 0.283 | 29.4 |
| 20 mg/L | 0.742 | 163.49 | 5.5 | 0.478 | 11.9 |
| Modified Gompertz Model | ||||||
|---|---|---|---|---|---|---|
| Chemical | ||||||
| Samples | R2 | PredictedP(mL/gVS) | Differences% | RmaxmL/gVS.day | λ (day) | RMSE |
| (4 mm) | 0.991 | 279.03 | 0.01 | 1.46 | 0.736 | 6.24 |
| (0.4 mm) | 0.994 | 340.28 | 0.08 | 1.45 | 0.785 | 5.77 |
| 5 mg/L | 0.995 | 369.73 | 0.87 | 1.60 | 0.651 | 4.58 |
| 10 mg/L | 0.991 | 375.09 | 2.57 | 1.58 | 0.624 | 7.07 |
| 20 mg/L | 0.982 | 171.24 | 0.14 | 1.37 | 0.777 | 5.30 |
| Green | ||||||
| 5 mg/L | 0.994 | 377.47 | 0.66 | 1.58 | 0.653 | 5.11 |
| 10 mg/L | 0.988 | 381.93 | 2.20 | 1.60 | 0.852 | 8.23 |
| 20 mg/L | 0.962 | 166.12 | 3.98 | 1.11 | 0.900 | 7.29 |
| Logistic Function Model | ||||||
| Chemical | ||||||
| Samples | R2 | PredictedP(mL/gVS) | Differences% | RmaxmL/gVS.day | λ (day) | RMSE |
| (4 mm) | 0.981 | 278.16 | 0.30 | 2.05 | 0.736 | 9.3 |
| (0.4 mm) | 0.986 | 339.32 | 0.20 | 2.01 | 1.16 | 8.00 |
| 5 mg/L | 0.987 | 368.57 | 1.19 | 2.27 | 0.952 | 7.29 |
| 10 mg/L | 0.981 | 373.69 | 2.94 | 2.27 | 0.917 | 9.98 |
| 20 mg/L | 0.978 | 170.96 | 0.021 | 1.97 | 1.12 | 5.08 |
| Green | ||||||
| 5 mg/L | 0.987 | 376.40 | 0.37 | 2.26 | 0.951 | 7.05 |
| 10 mg/L | 0.976 | 380.14 | 2.65 | 2.31 | 0.863 | 11.52 |
| 20 mg/L | 0.953 | 165.89 | 4.11 | 1.63 | 1.25 | 7.55 |
| Cost | Units | Barley (4 mm) | Barley (0.4 mm) |
|---|---|---|---|
| Mechanical Treatment Cost for Barley Straw (chipping + milling) | €/ton | 0 | 13.72 |
| Chemicals (NPs + NaOH) | €/100g | 0 | 0 |
| Total | €/ha | 871.11 | 884.83 |
| Benefits | |||
| Electricity Production (40% eff) | KWh/ha | 969 | 1181 |
| Electricity Sales | €/ha | 62 | 76 |
| Total | €/ha | 62 | 76 |
| Net Benefits | €/ha | −809.11 | −808.83 |
| Cost | Units | 5mg/L (0.4 mm) | 10 mg/L (0.4 mm) | 20 mg/L (0.4 mm) |
|---|---|---|---|---|
| Mechanical Treatment Price Needed for Barley Straw (chipping + milling) | €/ton | 13.72 | 13.72 | 13.72 |
| Chemicals (NPs + NaOH) | €/100g | 5.52 | 5.52 | 5.52 |
| Total | €/ha | 890.35 | 890.35 | 890.35 |
| Benefits | ||||
| Electricity Production (40% eff) | KWh/ha | 1302 | 1356 | 601 |
| Electricity Sales | €/ha | 83 | 87 | 38 |
| Total | €/ha | 83 | 87 | 38 |
| Net Benefits | €/ha | −807.35 | −803.35 | −852.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassaan, M.A.; Pantaleo, A.; Santoro, F.; Elkatory, M.R.; De Mastro, G.; Sikaily, A.E.; Ragab, S.; Nemr, A.E. Techno-Economic Analysis of ZnO Nanoparticles Pretreatments for Biogas Production from Barley Straw. Energies 2020, 13, 5001. https://doi.org/10.3390/en13195001
Hassaan MA, Pantaleo A, Santoro F, Elkatory MR, De Mastro G, Sikaily AE, Ragab S, Nemr AE. Techno-Economic Analysis of ZnO Nanoparticles Pretreatments for Biogas Production from Barley Straw. Energies. 2020; 13(19):5001. https://doi.org/10.3390/en13195001
Chicago/Turabian StyleHassaan, Mohamed A., Antonio Pantaleo, Francesco Santoro, Marwa R. Elkatory, Giuseppe De Mastro, Amany El Sikaily, Safaa Ragab, and Ahmed El Nemr. 2020. "Techno-Economic Analysis of ZnO Nanoparticles Pretreatments for Biogas Production from Barley Straw" Energies 13, no. 19: 5001. https://doi.org/10.3390/en13195001