Electro-Fenton Based Technique to Enhance Cell Harvest and Lipid Extraction from Microalgae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Culture Conditions
2.2. Neutral Lipid Extraction and Fatty Acid Analysis
2.3. Cell Disruption
2.4. Optimization of Electro-Fenton Conditions
2.5. Hydroxyl Radical Detection
2.6. Iron Detection
2.7. Electron Microscopy and Fluorescence Microscope Analysis
2.8. Reuse of Electro-Fenton Treated Microalgal Culture Medium for Subsequent Cultivation of Algae
3. Results and Discussion
3.1. Growth Properties
3.2. The Effects of Different Factors on Electro-Fenton Treatment for Lipid Extraction
3.3. Confirmation of Predicted Optimum Condition
3.4. Fatty Acid (FA) Composition Analysis during Electro-Fenton Reaction
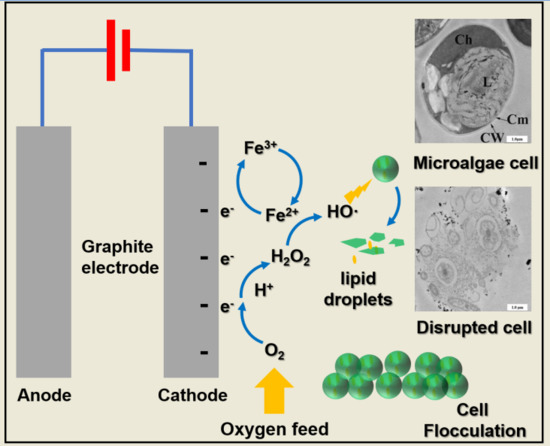
3.5. Microalgae Cell Disruption Mechanism in the Electro-Fenton Reaction
3.5.1. Hydroxyl Radical Generation in the Electro-Fenton Reaction
3.5.2. TEM for Microalgae Cell Wall and Cytomembrane Degradation in Electro-Fenton Reaction
3.5.3. Fluorescence Microscope (FM) for Microalgae Observation during Electro-Fenton Reaction
3.6. Iron Distribution
3.7. Microalgae Cultivation in the Electro-Fenton Wastewater
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Demirbas, M.F. Biorefineries for biofuel upgrading: A critical review. Appl. Energy 2009, 86, S151–S161. [Google Scholar] [CrossRef]
- Moreira, D.; Pires, J.C. Atmospheric CO2 capture by algae: Negative carbon dioxide emission path. Bioresour. Technol. 2016, 215, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Concas, A.; Lutzu, G.A.; Pisu, M.; Cao, G. Experimental analysis and novel modeling of semi-batch photobioreactors operated with Chlorella vulgaris and fed with 100% (v/v) CO2. Chem. Eng. J. 2012, 213, 203–213. [Google Scholar] [CrossRef]
- Concas, A.; Steriti, A.; Pisu, M.; Cao, G. Comprehensive modeling and investigation of the effect of iron on the growth rate and lipid accumulation of Chlorella vulgaris cultured in batch photobioreactors. Bioresour. Technol. 2014, 153, 340–350. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Yu, C.; Yin, Y.; Zhou, G. Evaluation of the potential of 9 Nannochloropsis strains for biodiesel production. Bioresour. Technol. 2014, 167, 503–509. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Rodrigues, C.M.; Pires, J.C.M.; Simões, M. The effect of increasing CO2 concentrations on its capture, biomass production and wastewater bioremediation by microalgae and cyanobacteria. Algal Res. 2016, 14, 127–136. [Google Scholar] [CrossRef]
- Wahlen, B.D.; Willis, R.M.; Seefeldt, L.C. Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed-cultures. Bioresour. Technol. 2011, 102, 2724–2730. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Ranjan, A.; Patil, C.; Moholkar, V.S. Mechanistic assessment of microalgal lipid extraction. Ind. Eng. Chem. Res. 2014, 49, 2979–2985. [Google Scholar] [CrossRef]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High Lipid Induction in Microalgae for Biodiesel Production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef] [Green Version]
- Wijffels, R.H.; Barbosa, M.J. An Outlook on Microalgal Biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäfer, K. Accelerated solvent extraction of lipids for determining the fatty acid composition of biological material. Anal. Chim. Acta 1998, 358, 69–77. [Google Scholar] [CrossRef]
- Cheng, C.H.; Du, T.B.; Pi, H.C.; Jang, S.M.; Lin, Y.H.; Lee, H.T. Comparative study of lipid extraction from microalgae by organic solvent and supercritical CO2. Bioresour. Technol. 2011, 102, 10151–10153. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.K.; Lewis, D.M.; Ashman, P.J. Disruption of microalgal cells for the extraction of lipids for biofuels: Processes and specific energy requirements. Biomass Bioenergy 2012, 46, 89–101. [Google Scholar] [CrossRef]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef]
- Halim, R.; Gladman, B.; Danquah, M.K.; Webley, P.A. Oil extraction from microalgae for biodiesel production. Bioresour. Technol. 2011, 102, 178–185. [Google Scholar] [CrossRef]
- Lardon, L.; Hélias, A.; Sialve, B.; Steyer, J.P.; Bernard, O. Life-cycle assessment of biodiesel production from microalgae. Environ. Sci. Technol. 2009, 43, 6475. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, A.; Shrestha, D.; McAloon, A.; Yee, W.; Haas, M.; Duffield, J.; Shapouri, H. Energy Life-Cycle assessment of soybean biodiesel revisited. Trans. ASABE 2009, 54, 1031–1039. [Google Scholar] [CrossRef]
- Xu, L.; Brilman, D.W.F.; Withag, J.A.M.; Brem, G.; Kersten, S. Assessment of a dry and a wet route for the production of biofuels from microalgae: Energy balance analysis. Bioresour. Technol. 2011, 102, 5113. [Google Scholar] [CrossRef]
- Taher, H.; Al-Zuhair, S.; Al-Marzouqi, A.H.; Haik, Y.; Farid, M. Effective extraction of microalgae lipids from wet biomass for biodiesel production. Biomass Bioenergy 2014, 66, 159–167. [Google Scholar] [CrossRef]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243. [Google Scholar] [CrossRef]
- Shirgaonkar, I.Z.; Lothe, R.R.; Pandit, A.B. Comments on the mechanism of microbial cell disruption in high-pressure and high-speed devices. Biotechnol. Prog. 1998, 14, 657–660. [Google Scholar] [CrossRef]
- Balasundaram, B.; Pandit, A.B. Selective release of invertase by hydrodynamic cavitation. Biochem. Eng. J. 2001, 8, 251–256. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, J.; Huang, Y.; Feng, J.; Zhou, J.; Cen, K. Dynamic microstructures and fractal characterization of cell wall disruption for microwave irradiation-assisted lipid extraction from wet microalgae. Bioresour. Technol. 2013, 150, 67. [Google Scholar] [CrossRef]
- Cheng, J.; Yu, T.; Li, T.; Zhou, J.; Cen, K. Using wet microalgae for direct biodiesel production via microwave irradiation. Bioresour. Technol. 2013, 131, 531–535. [Google Scholar] [CrossRef]
- Naghdi, F.G.; González, L.M.G.; Chan, W.; Schenk, P.M. Progress on lipid extraction from wet algal biomass for biodiesel production. Microb. Biotechnol. 2016, 9, 718–726. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. Application of cavitational reactors for cell disruption for recovery of intracellular enzymes. J. Chem. Technol. Biotechnol. 2008, 83, 1083–1093. [Google Scholar] [CrossRef]
- Keris-Sen, U.D.; Sen, U.; Soydemir, G.; Gurol, M.D. An investigation of ultrasound effect on microalgal cell integrity and lipid extraction efficiency. Bioresour. Technol. 2014, 152, 407–413. [Google Scholar] [CrossRef]
- Valizadeh Derakhshan, M.; Nasernejad, B.; Dadvar, M.; Hamidi, M. Pretreatment and kinetics of oil extraction from algae for biodiesel production. Asia Pac. J. Chem. Eng. 2014, 9, 629–637. [Google Scholar] [CrossRef]
- Grimi, N.; Dubois, A.; Marchal, L.; Jubeau, S.; Lebovka, N.I.; Vorobiev, E. Selective extraction from microalgae Nannochloropsis sp. using different methods of cell disruption. Bioresour. Technol. 2014, 153, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Vijayan, D.; Praveenkumar, R.; Han, J.-I.; Lee, K.; Park, J.-Y.; Chang, W.-S.; Lee, J.-S.; Oh, Y.-K. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310. [Google Scholar] [CrossRef]
- Park, J.Y.; Oh, Y.K.; Lee, J.S.; Lee, K.; Jeong, M.J.; Choi, S.A. Acid-catalyzed hot-water extraction of lipids from Chlorella vulgaris. Bioresour. Technol. 2014, 153, 408. [Google Scholar] [CrossRef]
- Kim, D.Y.; Oh, Y.K.; Park, J.Y.; Kim, B.; Choi, S.A.; Han, J.I. An integrated process for microalgae harvesting and cell disruption by the use of ferric ions. Bioresour. Technol. 2015, 191, 469–474. [Google Scholar] [CrossRef]
- Teixeira, R.E. Energy-efficient extraction of fuel and chemical feedstocks from algae. Green Chem. 2012, 14, 419–427. [Google Scholar] [CrossRef]
- Vanthoorkoopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef]
- Nurra, C.; Torras, C.; Clavero, E.; Ríos, S.; Rey, M.; Lorente, E.; Farriol, X.; Salvadó, J. Biorefinery concept in a microalgae pilot plant. Culturing, dynamic filtration and steam explosion fractionation. Bioresour. Technol. 2014, 163, 136. [Google Scholar] [CrossRef]
- Badawy, M.I.; Ali, M.E. Fenton’s peroxidation and coagulation processes for the treatment of combined industrial and domestic wastewater. J. Hazard. Mater. 2006, 136, 961. [Google Scholar] [CrossRef]
- Concas, A.; Pisu, M.; Cao, G. Disruption of microalgal cells for lipid extraction through Fenton reaction: Modeling of experiments and remarks on its effect on lipids composition. Chem. Eng. J. 2015, 263, 392–401. [Google Scholar] [CrossRef]
- Steriti, A.; Rossi, R.; Concas, A.; Cao, G. A novel cell disruption technique to enhance lipid extraction from microalgae. Bioresour. Technol. 2014, 164, 70–77. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Qiang, Z.; Chang, J.-H.; Huang, C.-P. Electrochemical generation of hydrogen peroxide from dissolved oxygen in acidic solutions. Water Res. 2002, 36, 85–94. [Google Scholar] [CrossRef]
- Lopes, G.K.; Schulman, H.M.; Hermeslima, M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. BBA Biomembr. 1999, 1472, 142–152. [Google Scholar]
- Oturan, M.A. An ecologically effective water treatment technique using electrochemically generated hydroxyl radicals for in situ destruction of organic pollutants: Application to herbicide 2, 4-D. J. Appl. Electrochem. 2000, 30, 475–482. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, Z.; Yue, Z.; Chen, S.; Zheng, Y.; Chen, F. CAH1 and CAH2 as key enzymes required for high bicarbonate tolerance of a novel microalga Dunaliella salina HTBS. Enzym. Microb. Technol. 2016, 87, 17–23. [Google Scholar] [CrossRef]
- Fajardo, A.R.; Cerdán, L.E.; Medina, A.R.; Moreno, P.A.G.; Grima, E.M. Lipid extraction from the microalga Phaeodactylum tricornutum. Eur. J. Lipid Sci. Technol. 2007, 109, 120–126. [Google Scholar] [CrossRef]
- Zhang, K.; Li, H.; Chen, W.; Zhao, M.; Cui, H.; Min, Q.; Wang, H.; Chen, S.; Li, D. Regulation of the Docosapentaenoic Acid/Docosahexaenoic Acid Ratio (DPA/DHA Ratio) in Schizochytrium limacinum B4D1. Appl. Biochem. Biotechnol. 2016, 182, 1–15. [Google Scholar] [CrossRef]
- Jen, J.F.; Leu, M.F.; Yang, T.C. Determination of hydroxyl radicals in an advanced oxidation process with salicylic acid trapping and liquid chromatography. J. Chromatogr. A 1998, 796, 283–288. [Google Scholar] [CrossRef]
- Gu, X.; Chen, C.; Zhou, T. Spectrophotometric method for the determination of ascorbic acid with iron (III)-1,10-phenanthroline after preconcentration on an organic solvent-soluble membrane filter. Anal. Bioanal. Chem. 1996, 355, 94–95. [Google Scholar] [CrossRef]
- Alemán-Nava, G.S.; Cuellar-Bermudez, S.P.; Cuaresma, M.; Bosma, R.; Muylaert, K.; Ritmann, B.E.; Parra, R. How to use Nile Red, a selective fluorescent stain for microalgal neutral lipids. J. Microbiol. Methods 2016, 128, 74–79. [Google Scholar] [CrossRef]
- Lieselot, B.; Cedrick, V.; Koen, G.; Charlotte, B.; Koenraad, M.; Imogen, F. Optimization of a Nile Red method for rapid lipid determination in autotrophic, marine microalgae is species dependent. J. Microbiol. Methods 2015, 118, 152. [Google Scholar]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell. Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Y.; Liu, C.F.; Hou, Y.Y.; Chen, S.L.; Xiao, D.G.; Zhang, J.K.; Chen, F.J. Isolation and Characterization of a Marine Microalga for Biofuel Production with Astaxanthin as a Co-Product. Energies 2013, 6, 2759–2772. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, M.; Oturan, N.; Dezotti, M.; Oturan, M.A. Phenol degradation by advanced electrochemical oxidation process electro-Fenton using a carbon felt cathode. Appl. Catal. B Environ. 2008, 83, 140–149. [Google Scholar] [CrossRef]
- Le, G.; Li, D.; Feng, G.; Liu, Z.; Hou, Y.; Chen, S.; Zhang, D. Hydroxyl radical-aided thermal pretreatment of algal biomass for enhanced biodegradability. Biotechnol. Biofuels 2015, 8, 194. [Google Scholar]
- Hua, L.; Guo, L.; Thakkar, M.; Wei, D.; Agbakpe, M.; Kuang, L.; Magpile, M.; Chaplin, B.P.; Tao, Y.; Shuai, D. Effects of anodic oxidation of a substoichiometric titanium dioxide reactive electrochemical membrane on algal cell destabilization and lipid extraction. Bioresour. Technol. 2016, 203, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995, 41, 1819. [Google Scholar] [CrossRef]
- Machlin, L.J.; Bendich, A. Free radical tissue damage: Protective role of antioxidant nutrients. FASEB J. 1987, 1, 441. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yeh, K.L.; Aisyah, R.; Lee, D.J.; Chang, J.S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef] [PubMed]







| Std. Order | Run Order | Fe2+ Conc. (mM) | Current Density (mA·cm−2) | Time (min) | Extraction Yield (wt%) |
|---|---|---|---|---|---|
| 17 | 1 | 10.00 | 17.50 | 30.00 | 84.00 |
| 11 | 2 | 10.00 | 5.00 | 60.00 | 64.97 |
| 6 | 3 | 15.00 | 17.50 | 0.00 | 43.31 |
| 3 | 4 | 5.00 | 30.00 | 30.00 | 58.41 |
| 10 | 5 | 10.00 | 30.00 | 0.00 | 40.36 |
| 12 | 6 | 10.00 | 30.00 | 60.00 | 54.80 |
| 13 | 7 | 10.00 | 17.50 | 30.00 | 81.71 |
| 16 | 8 | 10.00 | 17.50 | 30.00 | 84.99 |
| 4 | 9 | 15.00 | 30.00 | 30.00 | 46.60 |
| 15 | 10 | 10.00 | 17.50 | 30.00 | 88.60 |
| 14 | 11 | 10.00 | 17.50 | 30.00 | 85.64 |
| 5 | 12 | 5.00 | 17.50 | 0.00 | 46.27 |
| 7 | 13 | 5.00 | 17.50 | 60.00 | 66.28 |
| 1 | 14 | 5.00 | 5.00 | 30.00 | 57.75 |
| 2 | 15 | 15.00 | 5.00 | 30.00 | 56.11 |
| 9 | 16 | 10.00 | 5.00 | 0.00 | 41.67 |
| 8 | 17 | 15.00 | 17.50 | 60.00 | 46.92 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Hou, Y.; Liu, Z.; Ji, X.; Wu, D.; Wang, W.; Zhang, D.; Wang, W.; Chen, S.; Chen, F. Electro-Fenton Based Technique to Enhance Cell Harvest and Lipid Extraction from Microalgae. Energies 2020, 13, 3813. https://doi.org/10.3390/en13153813
Zhang S, Hou Y, Liu Z, Ji X, Wu D, Wang W, Zhang D, Wang W, Chen S, Chen F. Electro-Fenton Based Technique to Enhance Cell Harvest and Lipid Extraction from Microalgae. Energies. 2020; 13(15):3813. https://doi.org/10.3390/en13153813
Chicago/Turabian StyleZhang, Shuai, Yuyong Hou, Zhiyong Liu, Xiang Ji, Di Wu, Weijie Wang, Dongyuan Zhang, Wenya Wang, Shulin Chen, and Fangjian Chen. 2020. "Electro-Fenton Based Technique to Enhance Cell Harvest and Lipid Extraction from Microalgae" Energies 13, no. 15: 3813. https://doi.org/10.3390/en13153813