Structural, Textural, and Catalytic Properties of Ni-CexZr1−xO2 Catalysts for Methane Dry Reforming Prepared by Continuous Synthesis in Supercritical Isopropanol
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Textural Properties of Supports and Catalysts
3.2. Structural Properties of Supports and Catalysts
3.3. TEM Analysis
3.4. XPS Analysis
3.5. H2-TPR Analysis
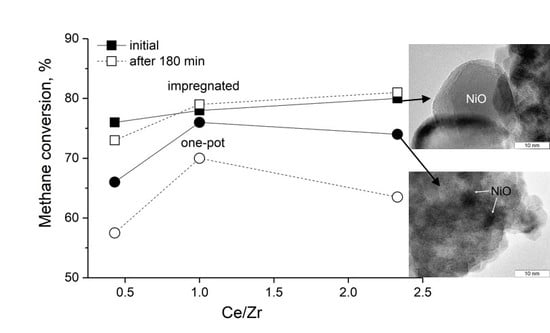
3.6. Catalytic Properties
3.7. TEM Analysis of Used Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, N.; Shojaee, M.; Spivey, J. Catalytic bi-reforming of methane: From greenhouse gases to syngas. Curr. Opin. Chem. Eng. 2015, 9, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Jang, W.-J.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Montoya, J.A. Methane reforming with CO2 over Ni/ZrO2–CeO2 catalysts prepared by sol–gel. Catal. Today 2000, 63, 71–85. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Q.; Zhang, J. Effect of preparation methods on structure and performance of Ni/Ce0.75Zr0.25O2 catalysts for CH4–CO2 reforming. Fuel 2008, 87, 2901–2907. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Romero-Nunez, A.; Diaz, G. High oxygen storage capacity and enhanced catalytic performance of NiO/NixCe1−xO2−δ nanorods: Synergy between Ni-doping and 1D morphology. RSC Adv. 2015, 5, 54571–54579. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Sophiphun, O.; Bernardi, J.; Wittayakun, J.; Föttinger, K.; Rupprechter, G. Methane dry reforming over ceria-zirconia supported Ni catalysts. Catal. Today 2016, 277, 234–245. [Google Scholar] [CrossRef] [Green Version]
- Roh, H.-S.; Potdar, H.; Jun, K.-W.; Kim, J.-W.; Oh, Y.-S. Carbon dioxide reforming of methane over Ni incorporated into Ce–ZrO2 catalysts. Appl. Catal. A Gen. 2004, 276, 231–239. [Google Scholar] [CrossRef]
- Kumar, P.; Sun, Y.; Idem, R.O. Nickel-Based Ceria, Zirconia, and Ceria–Zirconia Catalytic Systems for Low-Temperature Carbon Dioxide Reforming of Methane. Energy Fuels 2007, 21, 3113–3123. [Google Scholar] [CrossRef]
- Makri, M.; Vasiliades, M.A.; Petallidou, K.; Efstathiou, A.M. Effect of support composition on the origin and reactivity of carbon formed during dry reforming of methane over 5wt% Ni/Ce1−xMxO2−δ (M=Zr4+, Pr3+) catalysts. Catal. Today 2016, 259, 150–164. [Google Scholar] [CrossRef]
- Zonetti, P.C.; Letichevsky, S.; Gaspar, A.B.; Sousa-Aguiar, E.F.; Appel, L.G. The NixCe0.75Zr0.25−xO2 solid solution and the RWGS. Appl. Catal. A Gen. 2014, 475, 48–54. [Google Scholar] [CrossRef]
- Bacani, R.; Toscani, L.M.; Martins, T.S.; Fantini, M.C.A.; Lamas, D.G.; Larrondo, S.A. Synthesis and characterization of mesoporous NiO2/ZrO2-CeO2 catalysts for total methane conversion. Ceram. Int. 2017, 43, 7851–7860. [Google Scholar] [CrossRef]
- Barrio, L.; Kubacka, A.; Zhou, G.; Estrella, M.; Martínez-Arias, A.; Hanson, J.C.; Fernández-García, M.; Rodriguez, J.A. Unusual Physical and Chemical Properties of Ni in Ce1−xNixO2−y oxides: Structural Characterization and Catalytic Activity for the Water Gas Shift Reaction. J. Phys. Chem. C 2010, 114, 12689–12697. [Google Scholar] [CrossRef]
- Adachi, G.-Y.; Masui, T. Synthesis and modification of ceria-based materials. In Catalysis by Ceria and Related Materials; Trovarelli, A., Ed.; Imperial College Press: London, UK, 2002; pp. 51–83. [Google Scholar]
- Masui, T.; Fujiwara, K.; Peng, Y.; Sakata, T.; Machida, K.-I.; Mori, H.; Adachi, G.-Y. Characterization and catalytic properties of CeO2–ZrO2 ultrafine particles prepared by the microemulsion method. J. Alloy. Compd. 1998, 269, 116–122. [Google Scholar] [CrossRef]
- Trovarelli, A.; Zamar, F.; Llorca, J.; De Leitenburg, C.; Dolcetti, G.; Kiss, J.T. Nanophase Fluorite-Structured CeO2–ZrO2 Catalysts Prepared by High-Energy Mechanical Milling. J. Catal. 1997, 169, 490–502. [Google Scholar] [CrossRef]
- Aymonier, C.; Philippot, G.; Erriguible, A.; Marre, S. Playing with chemistry in supercritical solvents and the associated technologies for advanced materials by design. J. Supercrit. Fluids 2018, 134, 184–196. [Google Scholar] [CrossRef]
- Darr, J.; Zhang, J.; Makwana, N.M.; Weng, X. Continuous Hydrothermal Synthesis of Inorganic Nanoparticles: Applications and Future Directions. Chem. Rev. 2017, 117, 11125–11238. [Google Scholar] [CrossRef] [Green Version]
- Adschiri, T.; Hakuta, Y.; Arai, K. Hydrothermal Synthesis of Metal Oxide Fine Particles at Supercritical Conditions. Ind. Eng. Chem. Res. 2000, 39, 4901–4907. [Google Scholar] [CrossRef]
- Cabañas, A.; Darr, J.A.; Lester, E.; Poliakoff, M. Continuous hydrothermal synthesis of inorganic materials in a near-critical water flow reactor; the one-step synthesis of nano-particulate Ce1−xZrxO2 (x = 0–1) solid solutions. J. Mater. Chem. 2001, 11, 561–568. [Google Scholar] [CrossRef]
- Kim, J.-R.; Myeong, W.-J.; Ihm, S.-K. Characteristics in oxygen storage capacity of ceria–zirconia mixed oxides prepared by continuous hydrothermal synthesis in supercritical water. Appl. Catal. B Environ. 2007, 71, 57–63. [Google Scholar] [CrossRef]
- Kim, J.-R.; Myeong, W.-J.; Ihm, S.-K. Characteristics of CeO2–ZrO2 mixed oxide prepared by continuous hydrothermal synthesis in supercritical water as support of Rh catalyst for catalytic reduction of NO by CO. J. Catal. 2009, 263, 123–133. [Google Scholar] [CrossRef]
- Zhang, J.; Ohara, S.; Umetsu, M.; Naka, T.; Hatakeyama, Y.; Adschiri, T. Colloidal Ceria Nanocrystals: A Tailor-Made Crystal Morphology in Supercritical Water. Adv. Mater. 2007, 19, 203–206. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y.-S.; Veriansyah, B.; Kim, J.-D.; Lee, Y.-W. Continuous Synthesis of Surface-Modified Metal Oxide Nanoparticles Using Supercritical Methanol for Highly Stabilized Nanofluids. Chem. Mater. 2008, 20, 6301–6303. [Google Scholar] [CrossRef]
- Veriansyah, B.; Park, H.; Kim, J.-D.; Min, B.K.; Shin, Y.H.; Lee, Y.-W.; Kim, J. Characterization of surface-modified ceria oxide nanoparticles synthesized continuously in supercritical methanol. J. Supercrit. Fluids 2009, 50, 283–291. [Google Scholar] [CrossRef]
- Wang, P.; Kobiro, K. Synthetic versatility of nanoparticles: A new, rapid, one-pot, single-step synthetic approach to spherical mesoporous (metal) oxide nanoparticles using supercritical alcohols. Pure Appl. Chem. 2014, 86, 785–800. [Google Scholar] [CrossRef] [Green Version]
- Slostowski, C.; Marre, S.; Babot, O.; Toupance, T.; Aymonier, C. Near- and Supercritical Alcohols as Solvents and Surface Modifiers for the Continuous Synthesis of Cerium Oxide Nanoparticles. Langmuir 2012, 28, 16656–16663. [Google Scholar] [CrossRef] [PubMed]
- Slostowski, C.; Marre, S.; Babot, O.; Toupance, T.; Aymonier, C. Effect of Thermal Treatment on the Textural Properties of CeO2Powders Synthesized in Near- and Supercritical Alcohols. ChemPhysChem 2015, 16, 3493–3499. [Google Scholar] [CrossRef]
- Smirnova, M.Y.; Pavlova, S.N.; Krieger, T.A.; Bespalko, Y.N.; Anikeev, V.I.; Chesalov, Y.A.; Kaichev, V.V.; Mezentseva, N.V.; Sadykov, V.A. The synthesis of Ce1–xZrxO2 oxides in supercritical alcohols and catalysts for carbon dioxide reforming of methane on their basis. Russ. J. Phys. Chem. B 2017, 11, 1312–1321. [Google Scholar] [CrossRef]
- Smirnova, M.Y.; Bobin, A.S.; Pavlova, S.N.; Ishchenko, A.V.; Selivanova, A.; Kaichev, V.; Cherepanova, S.V.; Krieger, T.A.; Arapova, M.V.; Roger, A.-C.; et al. Methane dry reforming over Ni catalysts supported on Ce–Zr oxides prepared by a route involving supercritical fluids. Open Chem. 2017, 15, 412–425. [Google Scholar] [CrossRef]
- Anikeev, V.I. Hydrothermal synthesis of metal oxide nano- and microparticles in supercritical water. Russ. J. Phys. Chem. A 2011, 85, 377–382. [Google Scholar] [CrossRef]
- Lamas, D.G.; Lascalea, G.E.; Juárez, R.E.; Djurado, E.; Pérez, L.; De Reca, N.E.W. Metastable forms of the tetragonal phase in compositionally homogeneous, nanocrystalline zirconia–ceria powders synthesised by gel-combustion. J. Mater. Chem. 2003, 13, 904–910. [Google Scholar] [CrossRef]
- Varez, A.; Garcia-Gonzalez, E.; Jolly, J.; Sanz, J. Structural characterization of Ce1−xZrxO2 (0≤ x≤ 1) samples prepared at 1650 °C by solid state reaction: A combined TEM and XRD study. J. Eur. Ceram. Soc. 2007, 27, 3677–3682. [Google Scholar] [CrossRef]
- Scofield, J. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Sing, K.S.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Lamas, D.; Fuentes, R.O.; Fábregas, I.O.; De Rapp, M.E.F.; Lascalea, G.E.; Casanova, J.R.; De Reca, N.E.W.; Craievich, A.F. Synchrotron X-ray diffraction study of the tetragonal–cubic phase boundary of nanocrystalline ZrO 2 –CeO 2 synthesized by a gel-combustion process. J. Appl. Crystallogr. 2005, 38, 867–873. [Google Scholar] [CrossRef]
- Yashima, M.; Arashi, H.; Kakihana, M.; Yoshimura, M. Raman Scattering Study of Cubic-Tetragonal Phase Transition in Zr1−xCexO2 Solid Solution. J. Am. Ceram. Soc. 1994, 77, 1067–1071. [Google Scholar] [CrossRef]
- Kim, D.-J.; Jung, H.-J.; Yang, I.-S. Raman Spectroscopy of Tetragonal Zirconia Solid Solutions. J. Am. Ceram. Soc. 1993, 76, 2106–2108. [Google Scholar] [CrossRef]
- Mahammadunnisa, S.; Manoj Kumar Reddy, P.; Lingaiah, N.; Subrahmanyam, C. NiO/Ce1−xNixO2−δ as an alternative to noble metal catalysts for CO oxidation. Catal. Sci. Technol. 2013, 3, 730–736. [Google Scholar] [CrossRef]
- Koubaissy, B.; Pietraszek, A.; Roger, A.-C.; Kiennemann, A. CO2 reforming of methane over Ce-Zr-Ni-Me mixed catalysts. Catal. Today 2010, 157, 436–439. [Google Scholar] [CrossRef]
- Alders, D.; Voogt, F.C.; Hibma, T.; Sawatzky, G.A. Nonlocal screening effects in 2 p x-ray photoemission spectroscopy of NiO (100). Phys. Rev. B 1996, 54, 7716–7719. [Google Scholar] [CrossRef]
- Carley, A.; Jackson, S.; O’Shea, J.; Roberts, M. The formation and characterisation of Ni3+—an X-ray photoelectron spectroscopic investigation of potassium-doped Ni(110)–O. Surf. Sci. 1999, 440, L868–L874. [Google Scholar] [CrossRef]
- Kosova, N.V.; Devyatkina, E.; Kaichev, V. Mixed layered Ni–Mn–Co hydroxides: Crystal structure, electronic state of ions, and thermal decomposition. J. Power Sources 2007, 174, 735–740. [Google Scholar] [CrossRef]
- Kaichev, V.; Teschner, D.; Saraev, A.A.; Kosolobov, S.; Gladky, A.; Prosvirin, I.P.; Rudina, N.; Ayupov, A.; Blume, R.; Havecker, M.; et al. Evolution of self-sustained kinetic oscillations in the catalytic oxidation of propane over a nickel foil. J. Catal. 2016, 334, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Daza, C.; Gamba, O.A.; Hernández, Y.; Centeno, M.Á.; Mondragón, F.; Moreno, S.; Molina, R. High-Stable Mesoporous Ni-Ce/Clay Catalysts for Syngas Production. Catal. Lett. 2011, 141, 1037–1046. [Google Scholar] [CrossRef]
- Wrobel, G.; Sohier, M.; D’Huysser, A.; Bonnelle, J.; Marcq, J. Hydrogenation catalysts based on nickel and rare earth oxides. Appl. Catal. A Gen. 1993, 101, 73–93. [Google Scholar] [CrossRef]
- Bernal, S.; Calvino, J.J.; Cauqui, M.A.; Gatica, J.; Cartes, C.L.; Pérez-Omil, J.A.; Pintado, J. Some contributions of electron microscopy to the characterisation of the strong metal–support interaction effect. Catal. Today 2003, 77, 385–406. [Google Scholar] [CrossRef]
- Hosokawa, S.; Imamura, S.; Iwamoto, S.; Inoue, M. Synthesis of CeO2–ZrO2 solid solution by glycothermal method and its oxygen release capacity. J. Eur. Ceram. Soc. 2011, 31, 2463–2470. [Google Scholar] [CrossRef] [Green Version]
- Pojanavaraphan, C.; Luengnaruemitchai, A.; Gulari, E. Effect of catalyst preparation on Au/Ce1−xZrxO2 and Au–Cu/Ce1−xZrxO2 for steam reforming of methanol. Int. J. Hydrogen Energy 2013, 38, 1348–1362. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Z.; Xu, J.; Zheng, J.; Liu, J.; Jiang, G.; Duan, A.; He, H. Comparative study on the preparation, characterization and catalytic performances of 3DOM Ce-based materials for the combustion of diesel soot. Appl. Catal. B Environ. 2011, 107, 302–315. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, J.; Zhao, Z.; Duan, A.; Jiang, G. The catalysts of three-dimensionally ordered macroporous Ce1−xZrxO2-supported gold nanoparticles for soot combustion: The metal–support interaction. J. Catal. 2012, 287, 13–29. [Google Scholar] [CrossRef]
- Tang, C.; Li, J.; Yao, X.; Sun, J.; Cao, Y.; Zhang, L.; Gao, F.; Deng, Y.; Dong, L. Mesoporous NiO–CeO2 catalysts for CO oxidation: Nickel content effect and mechanism aspect. Appl. Catal. A Gen. 2015, 494, 77–86. [Google Scholar] [CrossRef]
- Pino, L.; Vita, A.; Cipitì, F.; Laganà, M.; Recupero, V. Catalytic Performance of Ce1−xNixO2 Catalysts for Propane Oxidative Steam Reforming. Catal. Lett. 2007, 122, 121–130. [Google Scholar] [CrossRef]
- Zhang, T.; Amiridis, M.D. Hydrogen production via the direct cracking of methane over silica-supported nickel catalysts. Appl. Catal. A Gen. 1998, 167, 161–172. [Google Scholar] [CrossRef]
- San-José-Alonso, D.; Juan-Juan, J.; Illán-Gómez, M.; Román-Martínez, M. Ni, Co and bimetallic Ni–Co catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2009, 371, 54–59. [Google Scholar] [CrossRef]
- Villacampa, J.I.; Royo, C.; Romeo, E.; Montoya, J.A.; Del Angel, P.; Monzón, A. Catalytic decomposition of methane over Ni-Al2O3 coprecipitated catalysts: Reaction and regeneration studies. Appl. Catal. A Gen. 2003, 252, 363–383. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. CO2 Reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Matte, L.P.; Kilian, A.S.; Luza, L.; Alves, M.D.C.; Morais, J.; Baptista, D.L.; Dupont, J.; Bernardi, F. Influence of the CeO2 Support on the Reduction Properties of Cu/CeO2 and Ni/CeO2 Nanoparticles. J. Phys. Chem. C 2015, 119, 26459–26470. [Google Scholar] [CrossRef]
- Caballero, A.; Holgado, J.P.; Gonzalez-Delacruz, V.M.; Habas, S.; Herranz, T.; Salmeron, M. In situ spectroscopic detection ofSMSI effect in a Ni/CeO2 system: Hydrogen-induced burial and dig out of metallic nickel. Chem. Commun. 2010, 46, 1097–1099. [Google Scholar] [CrossRef] [Green Version]














| Sample | SSA (m2∙g−1) | Vpore (cm3∙g−1) | Vmicropore (cm3∙g−1) | D 1 (nm) |
|---|---|---|---|---|
| Ce0.3Zr0.7 | 32 | 0.052 | 0.0013 | 9.9 |
| Ce0.5Zr0.5 | 42 | 0.047 | 0.0002 | 5.6 |
| Ce0.7Zr0.3 | 66 | 0.090 | 0.0033 | 7.5 |
| Ni-Ce0.3Zr0.7im | 20 | n.d. | n.d. | n.d. |
| Ni-Ce0.5Zr0.5im | 23 | n.d. | n.d. | n.d. |
| Ni-Ce0.7Zr0.3im | 50 | n.d. | n.d. | n.d. |
| Ni-Ce0.3Zr0.7op | 29 | 0.045 | 0 | 9.8 |
| Ni-Ce0.5Zr0.5op | 25 | 0.045 | 0.0011 | 16.9 |
| Ni-Ce0.7Zr0.3op | 36 | 0.060 | 0.0018 | 8.9 |
| Sample | a (Å) | c (Å) | c/a’ 1 | V | D (nm) | N(Ce) 2 | W 3 (%) |
|---|---|---|---|---|---|---|---|
| Ce0.3Zr0.7 | 3.686(7) | 5.291(1) | 1.015 | 71.9 | 14 | 0.34 | 100 |
| Ce0.5Zr0.5 | 3.754(1) | 5.335(2) | 1.005 | 75.19 | 14 | 0.59 | 93 |
| 3.674(3) | 5.27(1) | 1.014 | 71.16 | 10 | 0.28 | 4 | |
| 5.402(3) | - | - | 157.7 | 11 | 0.94 | 3 | |
| Ce0.7Zr0.3 | 3.780(1) | 5.372(2) | 1.005 | 76.75 | 14 | 0.71 | 86 |
| 5.41(1) | - | - | 158.3 | 14 | 0.97 | 14 | |
| Ni-Ce0.3Zr0.7op | 3.677(1) | 5.277(1) | 1.015 | 71.3 | 10 | 0.29 | 100 |
| Ni-Ce0.5Zr0.5op | 3.748(1) | 5.326(1) | 1.005 | 74.8 | 14 | 0.56 | 98 |
| 5.405(3) | - | - | 157.9 | 11 | 0.95 | 2 | |
| Ni-Ce0.7Zr0.3op | 3.78(2) | 5.36(7) | 1.003 | 76.6 | 14 | 0.70 | 86 |
| 5.410(1) | - | - | 158.3 | 13 | 0.97 | 14 |
| Catalyst | Ce/Zr | [Ni]/([Ce]+[Zr]) | C(NiO)/C(Ni*) |
|---|---|---|---|
| Ni-Ce0.3Zr0.7im | 0.33 | 0.19 | 100/0 |
| Ni-Ce0.3Zr0.7im reduced | 0.37 | 0.21 | 100/0 |
| Ni-Ce0.3Zr0.7op | 0.22 | 0.06 | 0/100 |
| Ni-Ce0.3Zr0.7op reduced | 0.27 | 0.05 | 0/100 |
| Ni-Ce0.7Zr0.3im | 2.7 | 0.19 | 100/0 |
| Ni-Ce0.7Zr0.3im reduced | 1.9 | 0.15 | 80/20 |
| Ni-Ce0.7Zr0.3op | 3.4 | 0.10 | 0/100 |
| Sample | H2 Consumption (mmol∙g−1) | ||||
|---|---|---|---|---|---|
| 100–300 °C | 300–700 °C | 100–700 °C | 100–900 °C | CeO2-ZrO21 | |
| Ce0.3Zr0.7 | 0 | 0.93 | 0.93 | 1.07 | |
| Ce0.5Zr0.5 | 0 | 0.94 | 0.94 | 1.40 | |
| Ce0.7Zr0.3 | 0 | 0.70 | 0.70 | 1.40 | |
| Ni-Ce0.3Zr0.7im | 0.08 | 1.59 | 1.67 | 1.83 | 0.98 |
| Ni-Ce0.5Zr0.5im | 0.08 | 1.46 | 1.75 | 2.04 | 1.19 |
| Ni-Ce0.7Zr0.3im | 0.25 | 1.41 | 1.66 | 2.22 | 1.37 |
| Ni-Ce0.3Zr0.7op | 0.24 | 1.59 | 1.83 | 2.09 | 1.24 |
| Ni-Ce0.5Zr0.5op | 0.68 | 1.23 | 1.9 | 2.38 | 1.53 |
| Ni-Ce0.7Zr0.3op | 0.64 | 0.97 | 1.7 | 2.17 | 1.32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlova, S.; Smirnova, M.; Bobin, A.; Cherepanova, S.; Kaichev, V.; Ishchenko, A.; Selivanova, A.; Rogov, V.; Roger, A.-C.; Sadykov, V. Structural, Textural, and Catalytic Properties of Ni-CexZr1−xO2 Catalysts for Methane Dry Reforming Prepared by Continuous Synthesis in Supercritical Isopropanol. Energies 2020, 13, 3728. https://doi.org/10.3390/en13143728
Pavlova S, Smirnova M, Bobin A, Cherepanova S, Kaichev V, Ishchenko A, Selivanova A, Rogov V, Roger A-C, Sadykov V. Structural, Textural, and Catalytic Properties of Ni-CexZr1−xO2 Catalysts for Methane Dry Reforming Prepared by Continuous Synthesis in Supercritical Isopropanol. Energies. 2020; 13(14):3728. https://doi.org/10.3390/en13143728
Chicago/Turabian StylePavlova, Svetlana, Marina Smirnova, Aleksei Bobin, Svetlana Cherepanova, Vasily Kaichev, Arcady Ishchenko, Aleksandra Selivanova, Vladimir Rogov, Anne-Cécile Roger, and Vladislav Sadykov. 2020. "Structural, Textural, and Catalytic Properties of Ni-CexZr1−xO2 Catalysts for Methane Dry Reforming Prepared by Continuous Synthesis in Supercritical Isopropanol" Energies 13, no. 14: 3728. https://doi.org/10.3390/en13143728