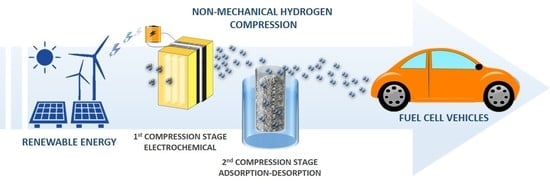
Towards Non-Mechanical Hybrid Hydrogen Compression for Decentralized Hydrogen Facilities
Abstract
:1. Introduction
2. Hydrogen as a Fuel
3. Hydrogen Storage for Automotive Applications
3.1. Compressed Hydrogen Tanks
3.2. Liquid Hydrogen
3.3. Solid Storage in Metal Hydrides
3.4. Solid Storage in Microporous Materials
4. The Hydrogen Value Chain
4.1. Centralised Hydrogen Distribution
4.2. Decentralized Facilities
5. Hydrogen Compression
5.1. Mechanical Hydrogen Compressors
5.2. Non-Mechanical Hydrogen Compressors
5.2.1. Metal Hydride Compressors
5.2.2. Electrochemical Compressors
5.2.3. Adsorption–Desorption Compressors
5.3. Overview of Costs and Efficiency
- uninstalled cost of the compressor system: USD 275,000
- specific energy consumption: 1.6 kWh kg−1
- annual maintenance costs: 4% of the uninstalled costs.
6. Non-Mechanical and Hybrid Hydrogen Compression in Decentralized Facilities
6.1. Performance of the Electrochemical Compressor
6.2. Performance of the Adsorption–Desorption Compressor
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Energy Agency. World Energy Balances 2019—Analysis. Available online: https://www.iea.org/reports/world-energy-balances-2019 (accessed on 2 May 2020).
- Dunn, S. Hydrogen futures: Toward a sustainable energy system. Int. J. Hydrog. Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- International Energy Agency. CO2 Emissions Statistics—Data Services. Available online: https://www.iea.org/subscribe-to-data-services/co2-emissions-statistics (accessed on 2 May 2020).
- NASA. Global Climate World of Change: Global Temperatures. Available online: https://earthobservatory.nasa.gov/world-of-change/global-temperatures (accessed on 2 May 2020).
- Thema, M.; Bauer, F.; Sterner, M. Power-to-Gas: Electrolysis and methanation status review. Renew. Sustain. Energy Rev. 2019, 112, 775–787. [Google Scholar] [CrossRef]
- Mazza, A.; Bompard, E.; Chicco, G. Applications of power to gas technologies in emerging electrical systems. Renew. Sustain. Energy Rev. 2018, 92, 794–806. [Google Scholar] [CrossRef]
- Gondal, I.A. Hydrogen integration in power-to-gas networks. Int. J. Hydrog. Energy 2019, 44, 1803–1815. [Google Scholar] [CrossRef]
- Hall, C.A.S.; Lambert, J.G.; Balogh, S.B. EROI of different fuels and the implications for society. Energy Policy 2014, 64, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Parra-Restrepo, J.; Bligny, R.; Dillet, J.; Didierjean, S.; Stemmelen, D.; Moyne, C.; Degiovanni, A.; Maranzana, G. Influence of the porous transport layer properties on the mass and charge transfer in a segmented PEM electrolyzer. Int. J. Hydrog. Energy 2020, 45, 8094–8106. [Google Scholar] [CrossRef]
- Babic, U.; Suermann, M.; Büchi, F.N.; Gubler, L.; Schmidt, T.J. Critical Review—Identifying Critical Gaps for Polymer Electrolyte Water Electrolysis Development. J. Electrochem. Soc. 2017, 164, F387–F399. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Ausiello, A.; Micoli, L.; Turco, M.; Toscano, G.; Florio, C.; Pirozzi, D. Biohydrogen production by dark fermentation of Arundo donax using a new methodology for selection of H2-producing bacteria. Int. J. Hydrog. Energy 2017, 42, 30599–30612. [Google Scholar] [CrossRef]
- Salkuyeh, Y.K.; Saville, B.A.; MacLean, H.L. Techno-economic analysis and life cycle assessment of hydrogen production from different biomass gasification processes. Int. J. Hydrog. Energy 2018, 43, 9514–9528. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen from solar energy, a clean energy carrier from a sustainable source of energy. Int. J. Energy Res. 2020, 44, 4110–4131. [Google Scholar] [CrossRef]
- He, W.; Namar, M.M.; Li, Z.; Maleki, A.; Tlili, I.; Safdari Shadloo, M. Thermodynamic analysis of a solar-driven high-temperature steam electrolyzer for clean hydrogen production. Appl. Therm. Eng. 2020, 172, 115152. [Google Scholar] [CrossRef]
- Karapekmez, A.; Dincer, I. Thermodynamic analysis of a novel solar and geothermal based combined energy system for hydrogen production. Int. J. Hydrog. Energy 2020, 45, 5608–5628. [Google Scholar] [CrossRef]
- Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Sol. Energy 2005, 78, 661–669. [Google Scholar] [CrossRef]
- Ahmed, M.; Dincer, I. A review on photoelectrochemical hydrogen production systems: Challenges and future directions. Int. J. Hydrog. Energy 2019, 44, 2474–2507. [Google Scholar] [CrossRef]
- International Energy Agency. Hydrogen—Tracking Energy Integration—Analysis. Available online: https://www.iea.org/reports/tracking-energy-integration/hydrogen (accessed on 2 June 2020).
- GlobalPetrolPrices.com. Gasoline Prices around the World. Available online: https://www.globalpetrolprices.com/gasoline_prices/ (accessed on 2 June 2020).
- Hydrogen Europe. Hydrogen in Industry. Available online: https://hydrogeneurope.eu/hydrogen-industry (accessed on 2 June 2020).
- Harvey, L.D.D. Energy and the New Reality 2: Carbon-free Energy Supply; Earthscan: New York, NY, USA, 2010; ISBN 978-1-84407-913-1. [Google Scholar]
- Commissariat de l’énergie atomique. L’hydrogène, les nouvelles technologies de l’énergie. Clefs CEA 2004, 50, 6. [Google Scholar]
- Amos, W.A. Costs of Storing and Transporting Hydrogen; National Renewable Energy Lab.: Golden, CO, USA, 1999.
- La Toyota. Mirai est la Voiture de Demain. Available online: https://www.toyota.fr/new-cars/new-mirai/landing.json (accessed on 15 April 2019).
- Krawczak, P. Réservoirs Haute Pression en Composites. Available online: https://www.techniques-ingenieur.fr/base-documentaire/materiaux-th11/applications-des-composites-42140210/reservoirs-haute-pression-en-composites-am5530/ (accessed on 17 June 2019).
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrog. Energy 2019. [Google Scholar] [CrossRef]
- James, B.D.; Houchins, C.; Huya-Kouadio, J.; DeSantis, D.A. Final Report: Hydrogen Storage System Cost Analysis; Strategic Analysis Inc.: Arlington, VA, USA, 2016. [Google Scholar]
- Fraser, D. Solutions for hydrogen storage and distribution. In Proceedings of the PEI Wind-Hydrogen Symposium, Charlottetown, PE, Canada, 22–24 June 2003. [Google Scholar]
- Michel, F.; Fieseler, H.; Allidières, L. Liquid hydrogen technologies for mobile use. In Proceedings of the World Hydrogen Energy Conference WHEC, Lyon, France, 13–16 June 2006. [Google Scholar]
- Liu, Z.; Li, Y. Thermal physical performance in liquid hydrogen tank under constant wall temperature. Renew. Energy 2019, 130, 601–612. [Google Scholar] [CrossRef]
- Sørensen, B.; Spazzafumo, G. 2—Hydrogen. In Hydrogen and Fuel Cells, 3rd ed.; Sørensen, B., Spazzafumo, G., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 5–105. ISBN 978-0-08-100708-2. [Google Scholar]
- Cardella, U.; Decker, L.; Klein, H. Roadmap to economically viable hydrogen liquefaction. Int. J. Hydrog. Energy 2017, 42, 13329–13338. [Google Scholar] [CrossRef]
- Selvam, P.; Viswanathan, B.; Swamy, C.S.; Srinivasan, V. Magnesium and magnesium alloy hydrides. Int. J. Hydrog. Energy 1986, 11, 169–192. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Alapati, S.V.; Johnson, J.K.; Sholl, D.S. Using first principles calculations to identify new destabilized metal hydride reactions for reversible hydrogen storage. Phys. Chem. Chem. Phys. 2007, 9, 1438–1452. [Google Scholar] [CrossRef] [PubMed]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrog. Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Department of Energy. Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles. Available online: https://energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles (accessed on 28 February 2017).
- Satyapal, S.; Petrovic, J.; Read, C.; Thomas, G.; Ordaz, G. The U.S. Department of Energy’s National Hydrogen Storage Project: Progress towards meeting hydrogen-powered vehicle requirements. Catal. Today 2007, 120, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; Bellosta von Colbe, J.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage—Past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Sheffield, J.W.; Martin, K.B.; Folkson, R. 5—Electricity and hydrogen as energy vectors for transportation vehicles. In Alternative Fuels and Advanced Vehicle Technologies for Improved Environmental Performance; Folkson, R., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 117–137. ISBN 978-0-85709-522-0. [Google Scholar]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353. [Google Scholar] [CrossRef]
- Davids, M.W.; Lototskyy, M.; Malinowski, M.; van Schalkwyk, D.; Parsons, A.; Pasupathi, S.; Swanepoel, D.; van Niekerk, T. Metal hydride hydrogen storage tank for light fuel cell vehicle. Int. J. Hydrog. Energy 2019. [Google Scholar] [CrossRef]
- Bellosta von Colbe, J.; Ares, J.-R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrog. Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Sdanghi, G.; Maranzana, G.; Celzard, A.; Fierro, V. Hydrogen Adsorption on Nanotextured Carbon Materials. In Hydrogen Storage Technologies; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 263–320. ISBN 978-1-119-46057-2. [Google Scholar]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.-W. Hydrogen storage in metal-organic frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, B.S.; Msayib, K.J.; McKeown, N.B.; M. Harris, K.D.; Pan, Z.; Budd, P.M.; Butler, A.; Selbie, J.; Book, D.; Walton, A. A triptycene-based polymer of intrinsic microposity that displays enhanced surface area and hydrogen adsorption. Chem. Commun. 2007, 0, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.M. Hydrogen adsorption and storage on porous materials. Catal. Today 2007, 120, 389–398. [Google Scholar] [CrossRef]
- Makhseed, S.; Samuel, J. Hydrogen adsorption in microporous organic framework polymer. Chem. Commun. 2008, 4342–4344. [Google Scholar] [CrossRef] [PubMed]
- Durette, D.; Bénard, P.; Zacharia, R.; Chahine, R. Investigation of the hydrogen adsorbed density inside the pores of MOF-5 from path integral grand canonical Monte Carlo at supercritical and subcritical temperature. Sci. Bull. 2016, 61, 594–600. [Google Scholar] [CrossRef] [Green Version]
- de la Casa-Lillo, M.A.; Lamari-Darkrim, F.; Cazorla-Amorós, D.; Linares-Solano, A. Hydrogen Storage in Activated Carbons and Activated Carbon Fibers. J. Phys. Chem. B 2002, 106, 10930–10934. [Google Scholar] [CrossRef]
- Fierro, V.; Zhao, W.; Izquierdo, M.T.; Aylon, E.; Celzard, A. Adsorption and compression contributions to hydrogen storage in activated anthracites. Int. J. Hydrog. Energy 2010, 35, 9038–9045. [Google Scholar] [CrossRef]
- Rzepka, M.; Lamp, P.; de la Casa-Lillo, M.A. Physisorption of Hydrogen on Microporous Carbon and Carbon Nanotubes. J. Phys. Chem. B 1998, 102, 10894–10898. [Google Scholar] [CrossRef] [Green Version]
- Farha, O.K.; Özgür Yazaydın, A.; Eryazici, I.; Malliakas, C.D.; Hauser, B.G.; Kanatzidis, M.G.; Nguyen, S.T.; Snurr, R.Q.; Hupp, J.T. De novo synthesis of a metal–organic framework material featuring ultrahigh surface area and gas storage capacities. Nat. Chem. 2010, 2, 944–948. [Google Scholar] [CrossRef]
- Cheng, H.-M.; Yang, Q.-H.; Liu, C. Hydrogen storage in carbon nanotubes. Carbon 2001, 39, 1447–1454. [Google Scholar] [CrossRef]
- Klebanoff, L.E.; Keller, J.O. 5 Years of hydrogen storage research in the U.S. DOE Metal Hydride Center of Excellence (MHCoE). Int. J. Hydrog. Energy 2013, 38, 4533–4576. [Google Scholar] [CrossRef]
- Schaefer, S.; Fierro, V.; Szczurek, A.; Izquierdo, M.T.; Celzard, A. Physisorption, chemisorption and spill-over contributions to hydrogen storage. Int. J. Hydrog. Energy 2016, 41, 17442–17452. [Google Scholar] [CrossRef]
- Zhao, W.; Fierro, V.; Fernández-Huerta, N.; Izquierdo, M.T.; Celzard, A. Hydrogen uptake of high surface area-activated carbons doped with nitrogen. Int. J. Hydrog. Energy 2013, 38, 10453–10460. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Fierro, V.; Zlotea, C.; Izquierdo, M.T.; Chevalier-César, C.; Latroche, M.; Celzard, A. Activated carbons doped with Pd nanoparticles for hydrogen storage. Int. J. Hydrog. Energy 2012, 37, 5072–5080. [Google Scholar] [CrossRef]
- Bossel, U.; Eliasson, B.; Taylor, G. The Future of the Hydrogen Economy: Bright or Bleak? Cogener. Compet. Power J. 2003, 18, 29–70. [Google Scholar] [CrossRef]
- Linde. Hydrogen. Available online: //www.linde-engineering.com/en/process_plants/hydrogen_and_synthesis_gas_plants/gas_products/hydrogen/index.html (accessed on 26 April 2019).
- McPhy. Electrolyseurs pour la Production Continue et Automatisée, et/ou en grande Quantité D’hydrogène. Available online: https://mcphy.com/fr/nos-produits-et-solutions/electrolyseurs/grande-capacite/ (accessed on 27 April 2019).
- Hydrogen Europe. Hydrogen Transport & Distribution. Available online: https://hydrogeneurope.eu/hydrogen-transport-distribution (accessed on 26 April 2019).
- Jancovici, J.-M. Que Peut-on Espérer des Piles à Combustible et de L’hydrogène? Available online: https://jancovici.com/transition-energetique/transports/que-peut-on-esperer-des-piles-a-combustible-et-de-l-hydrogene/ (accessed on 13 May 2019).
- Gerboni, R. 11—Introduction to hydrogen transportation. In Compendium of Hydrogen Energy; Woodhead Publishing Series in Energy; Gupta, R.B., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 283–299. ISBN 978-1-78242-362-1. [Google Scholar]
- US Department of Energy. 3.2 Hydrogen Delivery. In Fuel Cells Technologies Office Multi-Year Research, Development and Demonstration (MYRD&D) Plan; US Department of Energy: Washington, DC, USA, 2013. [Google Scholar]
- Yang, C.; Ogden, J. Determining the lowest-cost hydrogen delivery mode. Int. J. Hydrog. Energy 2007, 32, 268–286. [Google Scholar] [CrossRef] [Green Version]
- Engie. Comment est Transportée L’électricité? Available online: https://particuliers.engie.fr/electricite/conseils-electricite/comprendre-electricite/etapes-transport-electricite.html (accessed on 16 May 2019).
- Effori, E.; Moussaoui, H.; Monaco, F.; Sharma, R.K.; Debayle, J.; Gavet, Y.; Delette, G.; Larbi, G.S.; Siebert, E.; Vulliet, J.; et al. Reaction Mechanism and Impact of Microstructure on Performances for the LSCF-CGO Composite Electrode in Solid Oxide Cells. Fuel Cells 2019, 19, 429–444. [Google Scholar] [CrossRef]
- Vibhu, V.; Flura, A.; Rougier, A.; Nicollet, C.; Fourcade, S.; Hungria, T.; Grenier, J.-C.; Bassat, J.-M. Electrochemical ageing study of mixed lanthanum/praseodymium nickelates La2-xPrxNiO4+δ as oxygen electrodes for solid oxide fuel or electrolysis cells. J. Energy Chem. 2020, 46, 62–70. [Google Scholar] [CrossRef]
- Brauns, J.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy: A Review. Processes 2020, 8, 248. [Google Scholar] [CrossRef] [Green Version]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Mergel, J.; Carmo, M.; Fritz, D. Status on Technologies for Hydrogen Production by Water Electrolysis. In Transition to Renewable Energy Systems; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 423–450. ISBN 978-3-527-67387-2. [Google Scholar]
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Porembskiy, V.I.; Korobtsev, S.V.; Fateev, V.N.; Auprêtre, F.; Millet, P. High-pressure PEM water electrolysis and corresponding safety issues. Int. J. Hydrog. Energy 2011, 36, 2721–2728. [Google Scholar] [CrossRef]
- Suermann, M.; Pătru, A.; Schmidt, T.J.; Büchi, F.N. High pressure polymer electrolyte water electrolysis: Test bench development and electrochemical analysis. Int. J. Hydrog. Energy 2017, 42, 12076–12086. [Google Scholar] [CrossRef]
- Schalenbach, M.; Stolten, D. High-pressure water electrolysis: Electrochemical mitigation of product gas crossover. Electrochim. Acta 2015, 156, 321–327. [Google Scholar] [CrossRef]
- Torregrossa, M. Hydrogène: Air Liquide Inaugure une Nouvelle Station à Orly. Available online: https://www.automobile-propre.com/hydrogene-air-liquide-inaugure-nouvelle-station-orly/ (accessed on 27 April 2019).
- California Fuel Cell Partnership. Cost to Refill. Available online: https://cafcp.org/content/cost-refill (accessed on 3 June 2019).
- Prix des Carburants en France. Available online: https://www.prix-carburants.gouv.fr/ (accessed on 27 April 2019).
- Apostolou, D.; Enevoldsen, P.; Xydis, G. Supporting green Urban mobility—The case of a small-scale autonomous hydrogen refuelling station. Int. J. Hydrog. Energy 2019, 44, 9675–9689. [Google Scholar] [CrossRef]
- Mainka, J.; Vivian, R. Faire Rouler les Voitures Hydrogène à Base D’énergie Renouvelable. Available online: http://theconversation.com/faire-rouler-les-voitures-hydrogene-a-base-denergie-renouvelable-108536 (accessed on 17 May 2019).
- H2 Station Maps. Costs and Financing. Available online: https://h2stationmaps.com/costs-and-financing (accessed on 17 May 2019).
- Tarkowski, R. Underground hydrogen storage: Characteristics and prospects. Renew. Sustain. Energy Rev. 2019, 105, 86–94. [Google Scholar] [CrossRef]
- HyUnder. Assessment of the Potential, the Actors and Relevant Business Cases for Large Scale and Seasonal Storage of Renewable Electricity by Hydrogen Underground Storage in Europe; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Caglayan, D.G.; Weber, N.; Heinrichs, H.U.; Linßen, J.; Robinius, M.; Kukla, P.A.; Stolten, D. Technical potential of salt caverns for hydrogen storage in Europe. Int. J. Hydrog. Energy 2020, 45, 6793–6805. [Google Scholar] [CrossRef]
- Hydro-Pac. High Pressure Gas Compressors, Pumps and Related Products. Available online: http://www.hydropac.com/hydrogen-compression.html (accessed on 27 April 2019).
- Laurencelle, F. Développement d’un Compresseur D’Hydrogène Basé sur le Cyclage Thermique des Hydrures Métalliques. Ph.D. Thesis, Université du Québec à Trois-Rivières, Trois-Rivières, QC, Canada, 2007. [Google Scholar]
- Dwivedi, S.N. Design Considerations for High-Pressure Reciprocating Compressors for Refinery Services. In Proceedings of the International Compressor Engineering Conference, West Lafayette, IN, USA, 17–20 July 1990. [Google Scholar]
- Griffith, W.A.; Flanagan, E.B. Online, Continuous Monitoring Of Mechanical Condition And Performance For Critical Reciprocating Compressors. Tex. AM Univ. Turbomach. Lab. 2001. [Google Scholar] [CrossRef]
- Almasi, A. Latest practical notes and recent lessons learned on reciprocating compressors. Aust. J. Mech. Eng. 2016, 14, 138–150. [Google Scholar] [CrossRef]
- Sdanghi, G.; Maranzana, G.; Celzard, A.; Fierro, V. Review of the current technologies and performances of hydrogen compression for stationary and automotive applications. Renew. Sustain. Energy Rev. 2019, 102, 150–170. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, Y.; Chen, J.; Peng, X. Research on the flowrate and diaphragm movement in a diaphragm compressor for a hydrogen refueling station. Int. J. Hydrog. Energy 2016, 41, 14842–14851. [Google Scholar] [CrossRef]
- Jia, X.; Chen, J.; Wu, H.; Peng, X. Study on the diaphragm fracture in a diaphragm compressor for a hydrogen refueling station. Int. J. Hydrog. Energy 2016, 41, 6412–6421. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Env. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef] [Green Version]
- Plechkova, N.V.; Seddon, K.R. Ionic Liquids Completely UnCOILed: Critical Expert Overviews; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 978-1-118-83998-0. [Google Scholar]
- Lei, Z.; Dai, C.; Chen, B. Gas Solubility in Ionic Liquids. Chem. Rev. 2014, 114, 1289–1326. [Google Scholar] [CrossRef] [PubMed]
- Schluecker, E.; Szarvas, L.; Uerdingen, E. New developments in pumps and compressors using ionic liquids. ACHEMA Worldw. News 2008, 1, 5–7. [Google Scholar]
- Mayer, M. From Prototype to Serial Production. Manufacturing Hydrogen Fuelling Stations. In Proceedings of the A3PS Conference 2014, Vienna, Austria, 20–21 October 2014; Available online: http://www.a3ps.at/sites/default/files/conferences/2014/papers/01_linde_mayer.pdf (accessed on 10 March 2020).
- The Linde Group. The Hydrogen Technologies. The Ionic Compressor 90 MPa—IC90; Linde Group: Pullach, Germany, 2014. [Google Scholar]
- Pahwa, P.K.; Pahwa, G.K. Hydrogen Economy; The Energy and Resources Institute (TERI): New Delhi, India, 2014; ISBN 978-81-7993-504-0. [Google Scholar]
- Lüdtke, K.H. Process. Centrifugal Compressors: Basics, Function, Operation, Design, Application; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-662-09449-5. [Google Scholar]
- Jackson, S.B. Hydrogen Compression by Centrifugal Compressors. U.S. Patent 3,401,111, 10 September 1968. [Google Scholar]
- Witkowski, A.; Rusin, A.; Majkut, M.; Stolecka, K. Comprehensive analysis of hydrogen compression and pipeline transportation from thermodynamics and safety aspects. Energy 2017, 141, 2508–2518. [Google Scholar] [CrossRef]
- Nie, D.; Chen, X.; Fan, Z.; Wu, Q. Failure analysis of a slot-welded impeller of recycle hydrogen centrifugal compressor. Eng. Fail. Anal. 2014, 42, 1–9. [Google Scholar] [CrossRef]
- Parks, G.; Boyd, R.; Cornish, J.; Remick, R. Hydrogen Station Compression, Storage, and Dispensing Technical Status and Costs; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2014.
- Cox, K.E.; Williamson, K.D. Hydrogen: Its Technology and Implication: Implication of Hydrogen Energy; CRC Press: London, UK, 2018; Volume 5, ISBN 978-1-351-08175-7. [Google Scholar]
- Laurencelle, F.; Dehouche, Z.; Goyette, J.; Bose, T. Integrated electrolyser—Metal hydride compression system. Int. J. Hydrog. Energy 2006, 31, 762–768. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Yartys, V.A.; Pollet, B.G.; Bowman, R.C. Metal hydride hydrogen compressors: A review. Int. J. Hydrog. Energy 2014, 39, 5818–5851. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, H.; Li, H. A 70 MPa hydrogen-compression system using metal hydrides. Int. J. Hydrog. Energy 2011, 36, 9079–9085. [Google Scholar] [CrossRef]
- Stamatakis, E.; Zoulias, E.; Tzamalis, G.; Massina, Z.; Analytis, V.; Christodoulou, C.; Stubos, A. Metal hydride hydrogen compressors: Current developments & early markets. Renew. Energy 2018, 127, 850–862. [Google Scholar] [CrossRef]
- Moton, J.M.; James, B.D.; Colella, W.G. Advances in Electrochemical Compression of Hydrogen. In Proceedings of the ASME 2014 12th International Conference on Fuel Cell Science, Engineering and Technology, Boston, MA, USA, 30 June–2 July 2014. [Google Scholar]
- Trégaro, M.; Rhandi, M.; Druart, F.; Deseure, J.; Chatenet, M. Electrochemical hydrogen compression and purification versus competing technologies: Part II. Challenges in electrocatalysis. Chin. J. Catal. 2020, 41, 770–782. [Google Scholar] [CrossRef]
- Ströbel, R.; Oszcipok, M.; Fasil, M.; Rohland, B.; Jörissen, L.; Garche, J. The compression of hydrogen in an electrochemical cell based on a PE fuel cell design. J. Power Sources 2002, 105, 208–215. [Google Scholar] [CrossRef]
- Wang, Y.; Ruiz Diaz, D.F.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Pasierb, P.; Rekas, M. High-Temperature Electrochemical Hydrogen Pumps and Separators. Int. J. Electrochem. 2011, 2011, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Suermann, M.; Kiupel, T.; Schmidt, T.J.; Büchi, F.N. Electrochemical Hydrogen Compression: Efficient Pressurization Concept Derived from an Energetic Evaluation. J. Electrochem. Soc. 2017, 164, F1187–F1195. [Google Scholar] [CrossRef]
- Sdanghi, G.; Dillet, J.; Didierjean, S.; Fierro, V.; Maranzana, G. Feasibility of Hydrogen Compression in an Electrochemical System: Focus on Water Transport Mechanisms. Fuel Cells 2019, 20, 370–380. [Google Scholar] [CrossRef]
- HyET. Hydrogen Efficiency Technologies. Available online: http://www.hyet.nl/newsite/technology/working-principle (accessed on 15 March 2017).
- Rohland, B.; Eberle, K.; Ströbel, R.; Scholta, J.; Garche, J. Electrochemical hydrogen compressor. Electrochim. Acta 1998, 43, 3841–3846. [Google Scholar] [CrossRef]
- Wiebe, W.; Unwerth, T.V.; Schmitz, S. Hydrogen pump for hydrogen recirculation in fuel cell vehicles. E3S Web Conf. 2020, 155, 01001. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Lee, H.; Hwang, Y.; Radermacher, R.; Wang, C. Electrochemical compressor driven metal hydride heat pump. Int. J. Refrig. 2015, 60, 278–288. [Google Scholar] [CrossRef]
- Sdanghi, G.; Nicolas, V.; Mozet, K.; Schaefer, S.; Maranzana, G.; Celzard, A.; Fierro, V. A 70 MPa hydrogen thermally driven compressor based on cyclic adsorption-desorption on activated carbon. Carbon 2020, 161, 466–478. [Google Scholar] [CrossRef]
- Pierre, M.; Tapan, B. L’hydrogène; John Libbey Eurotext: Arcueil, France, 2006; ISBN 978-2-7420-1318-0. [Google Scholar]
- Rhandi, M.; Trégaro, M.; Druart, F.; Deseure, J.; Chatenet, M. Electrochemical hydrogen compression and purification versus competing technologies: Part I. Pros and cons. Chin. J. Catal. 2020, 41, 756–769. [Google Scholar] [CrossRef]
- Kadono, K.; Kajiura, H.; Shiraishi, M. Dense hydrogen adsorption on carbon subnanopores at 77 K. Appl. Phys. Lett. 2003, 83, 3392–3394. [Google Scholar] [CrossRef]
- Poirier, E.; Dailly, A. On the Nature of the Adsorbed Hydrogen Phase in Microporous Metal−Organic Frameworks at Supercritical Temperatures. Langmuir 2009, 25, 12169–12176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ting, V.P.; Ramirez-Cuesta, A.J.; Bimbo, N.; Sharpe, J.E.; Noguera-Diaz, A.; Presser, V.; Rudic, S.; Mays, T.J. Direct Evidence for Solid-like Hydrogen in a Nanoporous Carbon Hydrogen Storage Material at Supercritical Temperatures. ACS Nano 2015, 9, 8249–8254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Yang, H.; Bénard, P.; Chahine, R. Numerical study of thermal effects in cryo-adsorptive hydrogen storage tank. J. Renew. Sustain. Energy 2013, 5, 021414. [Google Scholar] [CrossRef]
- Wang, L.W.; Tamainot-Telto, Z.; Thorpe, R.; Critoph, R.E.; Metcalf, S.J.; Wang, R.Z. Study of thermal conductivity, permeability, and adsorption performance of consolidated composite activated carbon adsorbent for refrigeration. Renew. Energy 2011, 36, 2062–2066. [Google Scholar] [CrossRef]
- Corgnale, C.; Sulic, M. Techno-Economic Analysis of High-Pressure Metal Hydride Compression Systems. Metals 2018, 8, 469. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, E. Benchmark Analysis & Pre-feasibility study for the market penetration of Metal Hydride Hydrogen Compressor. In Proceedings of the Integrated, Innovative Renewable Energy—Hydrogen Systems and Applications Workshop, Athens, Greece, 5–7 July 2017. [Google Scholar]
- Cornish, A.J. Hydrogen Fueling Station Cost Reduction Study. In Survey Results and Analysis of the Cost and Efficiency of Various In-Operation Hydrogen Fueling Stations; Engineering, Procurement & Construction, LLC: Lakewood, CO, USA, 2011. Available online: https://www.osti.gov/servlets/purl/1120569 (accessed on 28 August 2019).
- Rand, D.A.J.; Dell, R.M.; Dell, R. Hydrogen Energy: Challenges and Prospects; RSC Publishing, Royal Society of Chemistry: Cambridge, UK, 2008; ISBN 978-0-85404-597-6. [Google Scholar]
- Hwang, H.T.; Varma, A. Hydrogen storage for fuel cell vehicles. Curr. Opin. Chem. Eng. 2014, 5, 42–48. [Google Scholar] [CrossRef]
- Toyota Europe. Hydrogen-Powered Toyota Mirai. Pioneering the Future of Mobility. Available online: https://www.toyota-europe.com/new-cars/mirai/ (accessed on 22 May 2019).
- Grigoriev, S.A.; Shtatniy, I.G.; Millet, P.; Porembsky, V.I.; Fateev, V.N. Description and characterization of an electrochemical hydrogen compressor/concentrator based on solid polymer electrolyte technology. Int. J. Hydrog. Energy 2011, 36, 4148–4155. [Google Scholar] [CrossRef]
- Schalenbach, M.; Hoefner, T.; Paciok, P.; Carmo, M.; Lueke, W.; Stolten, D. Gas Permeation through Nafion. Part 1: Measurements. J. Phys. Chem. C 2015, 119, 25145–25155. [Google Scholar] [CrossRef]
- Ramousse, J.; Deseure, J.; Lottin, O.; Didierjean, S.; Maillet, D. Modelling of heat, mass and charge transfer in a PEMFC single cell. J. Power Sources 2005, 145, 416–427. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Wood, C.D.; Bradshaw, D.; Rosseinsky, M.J.; Cooper, A.I. Hydrogen adsorption in microporous hypercrosslinked polymers. Chem. Commun. 2006, 2670–2672. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Kim, T.; Im, J.H.; Kim, Y.S.; Lee, K.; Jung, H.; Park, C.R. MOF-Derived Hierarchically Porous Carbon with Exceptional Porosity and Hydrogen Storage Capacity. Chem. Mater. 2012, 24, 464–470. [Google Scholar] [CrossRef]
- Cabria, I.; López, M.J.; Alonso, J.A. The optimum average nanopore size for hydrogen storage in carbon nanoporous materials. Carbon 2007, 45, 2649–2658. [Google Scholar] [CrossRef]
- Sdanghi, G.; Nicolas, V.; Mozet, K.; Maranzana, G.; Celzard, A.; Fierro, V. Modelling of a hydrogen thermally driven compressor based on cyclic adsorption-desorption on activated carbon. Int. J. Hydrog. Energy 2019, 44, 16811–16823. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Butler, B. An overview of development and challenges in hydrogen powered vehicles. Int. J. Green Energy 2020, 17, 13–37. [Google Scholar] [CrossRef] [Green Version]
- Celzard, A.; Fierro, V. Preparing a Suitable Material Designed for Methane Storage: A Comprehensive Report. Energy Fuels 2005, 19, 573–583. [Google Scholar] [CrossRef]
- Celzard, A.; Albiniak, A.; Jasienko-Halat, M.; Marêché, J.F.; Furdin, G. Methane storage capacities and pore textures of active carbons undergoing mechanical densification. Carbon 2005, 43, 1990–1999. [Google Scholar] [CrossRef]










| Characteristic | Piston | Diaphragm | Ionic Liquid |
|---|---|---|---|
| Compression Rate (Nm3 h−1) | ~10,000 | <1000 | <1000 |
| Efficiency | ~45% | ~45% | >70% |
| Cost | ~USD 170,000 1 | ~USD 2300 kg−1 day−1 | no data |
| Energy consumption (kWh kg−1) | <5 | <5 | ~2.7 |
| Advantages |
|
|
|
| Disadvantages |
|
|
|
| Characteristic | Metal Hydrides | Electrochemical | Adsorption–Desorption |
|---|---|---|---|
| Compression Rate (Nm3 h−1) | <10 | <10 | no data 2 |
| Efficiency (%) | <10 | ~60 | no data 2 |
| Cost (USD) | ~150,000 1 | ~170 kg−1 day−1 | no data 2 |
| Energy consumption (kWh kg−1) | 10 | <4 | no data 2 |
| Advantages |
|
|
|
| Disadvantages |
|
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sdanghi, G.; Maranzana, G.; Celzard, A.; Fierro, V. Towards Non-Mechanical Hybrid Hydrogen Compression for Decentralized Hydrogen Facilities. Energies 2020, 13, 3145. https://doi.org/10.3390/en13123145
Sdanghi G, Maranzana G, Celzard A, Fierro V. Towards Non-Mechanical Hybrid Hydrogen Compression for Decentralized Hydrogen Facilities. Energies. 2020; 13(12):3145. https://doi.org/10.3390/en13123145
Chicago/Turabian StyleSdanghi, Giuseppe, Gaël Maranzana, Alain Celzard, and Vanessa Fierro. 2020. "Towards Non-Mechanical Hybrid Hydrogen Compression for Decentralized Hydrogen Facilities" Energies 13, no. 12: 3145. https://doi.org/10.3390/en13123145