Experimental Investigation of Copper Mesh Substrate with Selective Wettability to Separate Oil/Water Mixture
Abstract
:1. Introduction
2. Experimental Platform
2.1. Materials
2.2. Sample Preparation
2.3. Instrumentation and Characterization
3. Characterizations and the Separation Results of the Membranes
3.1. Microstructure on the Surface of the Membranes
3.2. The Composition of the Prepared Membranes
3.3. Wettability of the Prepared Membranes
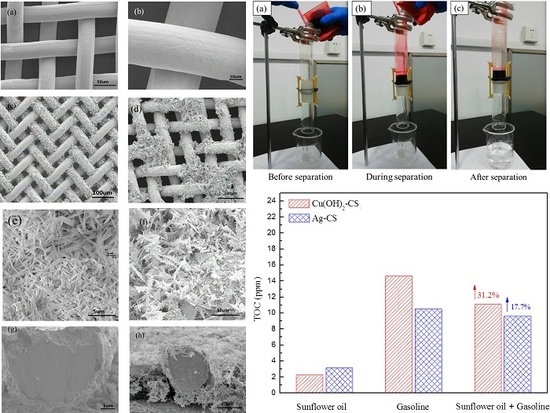
3.4. Single Component Oil/Water Mixture Separation
3.5. Multicomponent Oil/Water Mixtures Separation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cheryan, M.; Rajagopalan, N. Membrane processing of oily streams. Wastewater treatment and waste reduction. J. Membr. Sci. 1998, 151, 13–28. [Google Scholar] [CrossRef]
- Padaki, M.; Murali, R.S.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Chan, Y.J.; Chong, M.F.; Chung, L.L.; Hassell, D.G. A review on anaerobic-aerobic treatment of industrial and municipal wastewater. Chem. Eng. J. 2009, 155, 1–18. [Google Scholar] [CrossRef]
- Gao, C.; Sun, Z.; Kan, L.; Chen, Y.N.; Cao, Y.Z.; Zhang, S.Y.; Lin, F. Integrated oil separation and water purification by a double-layer TiO2-based mesh. Energy Environ. Sci. 2013, 6. [Google Scholar] [CrossRef]
- Vemu, S.; Pinnamaneni, U.B. Cross-flow microfiltration of industrial oily wastewater: Experimental and theoretical consideration. Sep. Sci. Technol. 2011, 46, 1213–1223. [Google Scholar]
- Lin, F.; Zhang, Z.Y.; Mai, Z.H.; Liu, B.Q.; Lei, J.; Zhu, D.B. A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water. Angew. Chem. Int. Ed. 2010, 43, 2012–2014. [Google Scholar]
- Ivshina, I.B.; Kuyukina, M.S.; Krivoruchko, A.V.; Elkin, A.A.; Makarov, S.O.; Cunningham, C.J.; Peshkur, T.A.; Atlas, R.M.; Philp, J.C. Oil spill problems and sustainable response strategies through new technologies. Environ. Sci. Process. Impacts 2015, 17, 1201–1219. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Wang, Y.; Long, Y.; Zhao, N.; Xu, J. Combination of bioinspiration: A general route to superhydrophobic particles. J. Am. Chem. Soc. 2012, 134. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Tai, N.H.; Lee, S.B.; Kuo, W.S. Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy. Environ. Sci. 2012, 7, 7908–7912. [Google Scholar] [CrossRef]
- Jenssen, B.M. Review article: Effects of oil pollution, chemically treated oil, and cleaning on thermal balance of birds. Environ. Pollut. 1994, 86. [Google Scholar] [CrossRef]
- Wang, S.; Song, Y.; Jiang, L. Microscale and nanoscale hierarchical structured mesh films with superhydrophobic and superoleophilic properties induced by long-chain fatty acids. Nanotechnology 2007, 18, 15103–15105. [Google Scholar] [CrossRef]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous materials for oil spill cleanup: A review of synthesis and absorbing properties. J. Porous Mat. 2003, 10, 59–170. [Google Scholar] [CrossRef]
- Srijaroonrat, P.; Julien, E.; Aurelle, Y. Unstable secondary oil/water emulsion treatment using ultrafiltration: Fouling control by backflushing. J. Membr. Sci. 1999, 159, 11–20. [Google Scholar] [CrossRef]
- Darling, S.B. Perspective: Interfacial materials at the interface of energy and water. J. Appl. Phys. 2018, 124. [Google Scholar] [CrossRef]
- Zhang, L.B.; Zhang, Z.H.; Peng, W. Smart surfaces with switchable superoleophilicity and superoleophobicity in aqueous media: Toward controllable oil/water separation. NPG Asia Mater. 2012, 4. [Google Scholar] [CrossRef]
- Crick, C.R.; Gibbins, J.A.; Parkin, I.P. Superhydrophobic polymer-coated copper-mesh; membranes for highly efficient oil-water separation. J. Mater. Chem. A 2013, 1. [Google Scholar] [CrossRef]
- Kammerer, M.; Mastain, O.; Dréan-Quenech’Du, S.L.; Pouliquen, H.; Larhantec, M. Liver and kidney concentrations of vanadium in oiled seabirds after the Erika wreck. Sci. Total Environ. 2004, 333, 295–301. [Google Scholar] [CrossRef]
- Kajitvichyanukul, P.; Hung, Y.T.; Wang, L.K. Oil Water Separation. Handbook of Environmental Engineering; Wang, L.K., Hung, Y.-T., Shammas, N.K., Eds.; Springer: Berlin, Germany, 2006. [Google Scholar]
- Kwon, W.T.; Park, K.; Han, S.D.; Yoon, S.M.; Kim, J.Y.; Bae, W.; Rhee, Y.W. Investigation of water separation from water-in-oil emulsion using electric field. J. Ind. Eng. Chem. 2010, 16, 684–687. [Google Scholar] [CrossRef]
- Eow, J.S.; Ghadiri, M.; Sharif, A.O. Electrostatic and hydrodynamic separation of aqueous drops in a flowing viscous oil. Chem. Eng. Process. Process Intensif. 2002, 41, 649–657. [Google Scholar] [CrossRef]
- Hanafy, M.; Nabih, H.I. Treatment of oily wastewater using dissolved air flotation technique. Energy Sources 2007, 29, 143–159. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Yang, Q.; Bian, H.; Du, G.Q.; Shan, C.; Huo, J.L.; Fang, Y.; Hou, H. Oil-Water Separation: Oil-Water Separation: A Gift from the Desert. Adv. Mater. Interfaces 2016, 3. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.Z.; Xu, X.H.; Zhu, X.T.; Men, X.H.; Zhou, X.Y. Superhydrophilic–superoleophobic coatings. J. Mater. Chem. 2012, 22, 2834–2837. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, W.B.; Shi, Z.; Wang, D.; Jin, J. Nanowire-Haired inorganic membranes with superhydrophilicity and underwater ultralow adhesive superoleophobicity for high-efficiency oil/water separation. Adv. Mater. 2013, 25, 4192–4198. [Google Scholar] [CrossRef]
- Xue, Z.X.; Liu, M.J.; Jiang, L. Recent developments in polymeric superoleophobic surfaces. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1209–1224. [Google Scholar] [CrossRef]
- Sun, T.L.; Lin, F.; Gao, X.F.; Jiang, L. Bioinspired surfaces with special wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, Y.N.; Xi, J.M.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal effect: A superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4114–4119. [Google Scholar]
- Feng, X.J.; Jiang, L. Design and creation of superwetting/antiwetting surfaces. Adv. Mat. 2010, 18, 3063–3078. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Xia, H.; Kim, E.Y.; Sun, H.B. Recent developments in superhydrophobic surfaces with unique structural and functional properties. Soft Matter 2012, 8, 11217. [Google Scholar] [CrossRef]
- Blossey, R. Self-cleaning surfaces—Virtual realities. Nat. Mat. 2003, 2, 301–306. [Google Scholar] [CrossRef]
- Zhang, J.; Seeger, S. Superoleophobic coatings with ultralow sliding angles based on silicone nanofilaments. Angew. Chem. Inte. Ed. 2011, 50, 6652–6656. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progess in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nature Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, F.; Niu, J.; Jiang, Z.Q. Superhydrophobic surfaces: From structural control to functional application. J. Mater. Chem. 2008, 18, 621–633. [Google Scholar] [CrossRef]
- Cheng, M.J.; Gao, Y.F.; Guo, X.P.; Shi, Z.Y.; Chen, J.F.; Shi, F. A functionally integrated device for effective and facile oil spill cleanup. Langmuir 2011, 27, 7371–7375. [Google Scholar] [CrossRef] [PubMed]
- Erbil, H.Y.; Demirel, A.L.; Avci, Y.; Mert, O. Transformation of a simple plastic into a superhydrophobic surface. Science 2003, 299, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.X.; Sun, Z.X.; Cao, Y.Z.; Chen, Y.N.; Tao, L.; Li, K.; Lin, F.; Fu, Q.; Wen, Y. Superoleophilic and superhydrophobic biodegradable material with porous structures for oil absorption and oil–water separation. RSC Adv. 2013, 3, 23432–23437. [Google Scholar] [CrossRef]
- Zhang, J.L.; Huang, W.H.; Han, Y.C. A composite polymer film with both superhydrophobicity and superoleophilicity. Macromol. Rapid Commun. 2010, 27, 804–808. [Google Scholar] [CrossRef]
- Tu, C.W.; Tsai, C.H.; Wang, C.F.; Kuo, S.W.; Chang, F.C. Fabrication of superhydrophobic and superoleophilic polystyrene surfaces by a facile one-step method. Macromol. Rapid Commun. 2010, 28, 2262–2266. [Google Scholar] [CrossRef]
- Choi, H.O.; Zhang, K.; Dionysiou, D.D. Effect of permeate flux and tangential flow on membrane fouling for wastewater treatment. Sep. Purif. Technol. 2015, 45, 68–78. [Google Scholar] [CrossRef]
- Huang, Y.; Zha, G.Y.; Luo, Q.J.; Zhang, J.X.; Zhang, F.; Li, X.H.; Zhao, S.F.; Zhu, W.P.; Li, X.D. The construction of hierarchical structure on Ti substrate with superior osteogenic activity and intrinsic antibacterial capability. Sci. Rep. 2014, 4, 6172. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.F.; He, A.H.; Li, J.X.; Han, C.C. Studies on the controlled morphology and wettability of polystyrene surfaces by electrospinning or electrospraying. Polymer 2006, 47, 7095–7102. [Google Scholar] [CrossRef]
- Wang, B.; Liang, W.X.; Guo, Z.G.; Liu, W.M. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Xie, Y.S.; Chan, H.; Narayanan, B.; Chen, L.; Waldman, R.Z.; Sankaranarayanan, S.K.R.S.; Elam, J.W.; Darling, S.B. Crude-oil-repellent membranes by atomic layer deposition: Oxide interface engineering. ACS Nano 2018, 12, 8678–8685. [Google Scholar] [CrossRef] [PubMed]
- Kota, A.K.; Kwon, G.B.; Choi, W.J. Hygro-responsive membranes for effective oil–water separation. Nat. Commun. 2012, 3, 1025. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.T.; Zhang, Z.Z.; Men, X.H.; Yang, J.; Wang, K.; Xu, X.H.; Zhou, X.Y.; Xue, Q.J. Robust superhydrophobic surfaces with mechanical durability and easy repairability. J. Mater. Chem. 2011, 21, 15793–15797. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]















© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Cui, C.; Qi, B.; Wei, J.; Qaisrani, M.A. Experimental Investigation of Copper Mesh Substrate with Selective Wettability to Separate Oil/Water Mixture. Energies 2019, 12, 4564. https://doi.org/10.3390/en12234564
Yuan J, Cui C, Qi B, Wei J, Qaisrani MA. Experimental Investigation of Copper Mesh Substrate with Selective Wettability to Separate Oil/Water Mixture. Energies. 2019; 12(23):4564. https://doi.org/10.3390/en12234564
Chicago/Turabian StyleYuan, Jia, Chenyi Cui, Baojin Qi, Jinjia Wei, and Mumtaz A. Qaisrani. 2019. "Experimental Investigation of Copper Mesh Substrate with Selective Wettability to Separate Oil/Water Mixture" Energies 12, no. 23: 4564. https://doi.org/10.3390/en12234564