The Impact of Substrate–Enzyme Proportion for Efficient Hydrolysis of Hay
Abstract
:1. Introduction
2. Materials and Methods
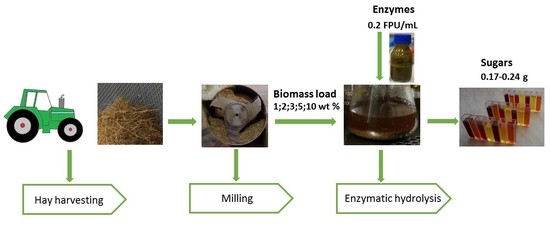
2.1. Biomass Pre-Treatment
2.2. Enzymatic Hydrolysis in Batch and Reactor Scale
2.3. Carbohydrate Analyses
2.4. Statistical Analyses
3. Results and Discussion
3.1. Adjustment of Reducing Sugar Measurements
3.2. Impact of Solid Loading on Hydrolysis Efficiency
3.3. Impact of Mixing and Enzyme Loading on Hydrolysis Efficiency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. Tracking Clean Energy Progress 2017; IEA Publications: Paris, France, 2017; pp. 1–116. [Google Scholar]
- Sanchez, O.J.; Montoya, S. Production of bioethanol from biomass: An overview. In Biofuel Technologies; Gupta, V.K., Tuohy, M.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 397–441. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: A European Strategy for Low-Emission Mobility; SWD (2016) 244 Final; European Commission: Brussels, Belgium, 20 July 2016.
- King, D. The Future of Industrial Biorefineries; World Economic Forum: Geneva, Switzerland, 2010. [Google Scholar]
- Jaramillo, P.M.D.; Gomes, H.A.R. Chapter 6. Lignocellulose-degrading enzymes: An overview of the global market. In Fungal Biomolecules: Sources, Applications and Recent Developments; Gupta, V.K., Mach, R.L., Sreenivasaprasad, S., Eds.; Wiley-Blackwell: Chichester, UK, 2015; pp. 75–86. [Google Scholar]
- Ghosh, D.; Dasgupta, D.; Agarwal, D.; Kaul, S.; Adhikari, D.K.; Kurmi, A.K.; Arya, P.K.; Bangwal, D.; Negi, M.S. Fuels and chemicals from lignocellulosic biomass: An integrated biorefinery approach. Energy Fuels 2015, 29, 3149–3157. [Google Scholar] [CrossRef]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pre-treatment, hydrolysis and fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products-strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, H.; Vibe-Pedersen, J.; Larsen, J.; Felby, C. Liquefaction of lignocellulose at high-solids concentrations. Biotechnol. Bioeng. 2007, 96, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Hagerdal, B.; Galbe, M.; Gorwa-Grauslund, M.F.; Liden, G.; Zacchi, G. Bio-ethanol—The fuel of tomorrow from the residues of today. Trends Biotechnol. 2006, 24, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Su, R.; Huang, R.; Qi, W.; He, Z. Co-optimization of sugar yield and input energy by the stepwise reduction of agitation rate during lignocellulose hydrolysis. Food Bioprod. Process. 2015, 95, 1–6. [Google Scholar] [CrossRef]
- Lou, H.; Zeng, M.; Hu, Q.; Cai, C.; Lin, X.; Qiu, X.; Yang, D.; Pang, Y. Nonionic surfactants enhanced enzymatic hydrolysis of cellulose by reducing cellulase deactivation caused by shear force and air-liquid interface. Bioresour. Technol. 2018, 249, 1–8. [Google Scholar] [CrossRef]
- Ghosh, S.; Chowdhury, R.; Bhattacharya, P. Sustainability of cereal straws for the fermentative production of second generation biofuels: A review of the efficiency and economics of biochemical pretreatment processes. Appl. Energy 2017, 198, 284–298. [Google Scholar] [CrossRef]
- Tutt, M.; Kikas, T.; Olt, J. Influence of different pre-treatment methods on bioethanol production from wheat straw. Agron. Res. 2012, 1, 269–276. [Google Scholar]
- Ketzer, D.; Rösch, C.; Haase, M. Assessment of sustainable grassland biomass potentials for energy supply in Northwest Europe. Biomass Bioenergy 2017, 100, 39–51. [Google Scholar] [CrossRef]
- Leclère, D.; Valin, H.; Frank, S.; Havlík, P. Assessing the Land Use Change Impacts of Using EU Grassland for Biofuel Production. Task 4b of Tender ENER/C1/2013; Project Number: BIENL15199; ECOFYS: Utrecht, The Netherlands, 2016; pp. 1–55. [Google Scholar]
- Monforti, F.; Lugato, E.; Montola, V.; Bodis, K.; Scarlat, N.; Dallemand, J.F. Optimal energy use of agricultural crop residues preserving soil organic carbon stocks in Europe. Renew. Sustain. Energy Rev. 2015, 44, 519–529. [Google Scholar] [CrossRef]
- Herrman, C.; Prochnow, A.; Heiermann, M.; Idler, C. Biomass from landscape management of grassland used for biogas production: Effects of harvest date and silage additives on feedstock quality and methane yield. Grass Forage Sci. 2013, 69, 549–566. [Google Scholar] [CrossRef]
- Mezule, L.; Dalecka, B.; Juhna, T. Fermentable Sugar Production from Lignocellulosic waste. Chem. Eng. Trans. 2015, 43, 619–624. [Google Scholar]
- Ghose, T.K. Measurement of cellulose activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- French, K.E. Assessing the bioenergy potential of grassland biomass from conservation areas in England. Land Use Policy 2019, 82, 700–708. [Google Scholar] [CrossRef]
- Tihomirova, K.; Dalecka, B.; Mezule, L. Application of conventional HPLC RI technique for sugar analysis in hydrolysed hay. Agron. Res. 2016, 14, 1713–1719. [Google Scholar]
- Gonçalves, C.; Rodriguez-Jasso, R.M.; Gomes, N.; Teixeira, J.A.; Belo, I. Adaptation of dinitrosalicylic acid method to microtiter plates. Anal. Methods 2010, 2, 2046–2048. [Google Scholar] [CrossRef] [Green Version]
- Garriga, M.; Almaraz, M.; Marchiaro, A. Determination of reducing sugars in extracts of Undaria pinnatifida (Harvey) algae by UV-visible spectrophotometry (DNS method). Actas Ing. 2017, 3, 173–179. [Google Scholar]
- Hodge, D.B.; Karim, M.N.; Schell, D.J.; McMillan, J.D. Soluble and insoluble solids contributions to high-solids enzymatic hydrolysis of lignocellulose. Bioresour. Technol. 2008, 99, 8940–8948. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Felby, C.; Jorgensen, H. Yield-determining factors in high-solids enzymatic hydrolysis of lignocellulose. Biotechnol. Biofuels 2009, 2, 1–11. [Google Scholar] [CrossRef]
- Kadić, A.; Palmqvist, B.; Lidén, G. Effects of agitation on particle-size distribution and enzymatic hydrolysis of pretreated spruce and giant reed. Biotechnol. Biofuels 2014, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.; Dragone, G.; Fernandes, M.; Milagres, A.; Roberto, I. The effect of agitation speed, enzyme loading and substrate concentration on enzymatic hydrolysis of cellulose from brewer’s spent grain. Cellulose 2008, 15, 711–721. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. Enzymatic hydrolysis of biomass at high-solids loadings—A review. Biomass Bioenergy 2013, 56, 526–544. [Google Scholar] [CrossRef]


| Glucose Standard, mg/mL | Single Calibration Curve Approach, mg/mL | Multiple Calibration Curve Approach, mg/mL |
|---|---|---|
| 2.5 | 2.29 | 2.47 * |
| 5.0 | 4.89 | 5.05 * |
| 7.0 | 6.24 | 6.62 * |
| 10.0 | 8.996 | 9.82 * |
| 13.0 | 11.69 | 13.08 * |
| 18.0 | 16.02 | 17.74 * |
| Solid Loading, wt% | 0.5 × Enzyme Concentration, 0.1 FPU/mL | Standard Concentration, 0.2 FPU/mL | Double Enzyme Concentration, 0.4 FPU/mL | |||
|---|---|---|---|---|---|---|
| I. lacteus | T. reesei | I. lacteus | T. reesei | I. lacteus | T. reesei | |
| 2 | 0.23 | 0.15 | 0.24 | 0.17 | 0.25 | 0.21 |
| 3 | 0.21 | 0.20 | 0.23 | 0.20 | 0.24 | 0.20 |
| 5 | 0.20 | 0.15 | 0.22 | 0.19 | 0.26 | 0.19 |
| 10 | 0.17 | 0.14 | 0.19 | 0.17 | 0.21 | 0.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezule, L.; Berzina, I.; Strods, M. The Impact of Substrate–Enzyme Proportion for Efficient Hydrolysis of Hay. Energies 2019, 12, 3526. https://doi.org/10.3390/en12183526
Mezule L, Berzina I, Strods M. The Impact of Substrate–Enzyme Proportion for Efficient Hydrolysis of Hay. Energies. 2019; 12(18):3526. https://doi.org/10.3390/en12183526
Chicago/Turabian StyleMezule, Linda, Ieva Berzina, and Martins Strods. 2019. "The Impact of Substrate–Enzyme Proportion for Efficient Hydrolysis of Hay" Energies 12, no. 18: 3526. https://doi.org/10.3390/en12183526