Life Cycle Assessment of Fuel Cell Vehicles Considering the Detailed Vehicle Components: Comparison and Scenario Analysis in China Based on Different Hydrogen Production Schemes
Abstract
:1. Introduction
1.1. Literature Review
1.2. Contribution of This Work
2. Methods
2.1. Assessment Objects and Data Sources
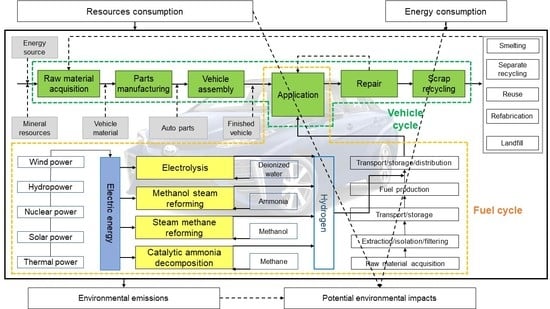
2.2. System Boundaries and Functional Units
2.3. Impact Assessment Indicators
2.4. Assessment Model
2.4.1. Assessment Model for the Vehicle Cycle
Comprehensive Resources and Energy Consumption of the Vehicle Cycle
Comprehensive Environmental Emissions of the Vehicle Cycle
2.4.2. Assessment Model for the Fuel Cycle
Comprehensive Resources and Energy Consumption of the Fuel Cycle
Comprehensive Environmental Emissions of the Fuel Cycle
2.4.3. Life Cycle Assessment Model of Toyota Mirai
Raw Material Acquisition Stage
Parts Manufacturing Stage
Vehicle Assembly Stage
Use Stage
Maintenance Stage
Scraping Recycling Stage
3. Results and Discussion
3.1. Energy Consumption and Emissions
3.2. Characterization Results
3.2.1. Mineral Resource Consumption
3.2.2. Fossil Energy Consumption
3.2.3. Environmental Emissions
3.3. Comparison of Four Hydrogen Production Schemes
3.3.1. Inventory Analysis and Data Collection
3.3.2. Impact Assessment and Result Analysis
3.4. Scenario Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix
| Steam Methane Reforming | Methanol Steam Reforming | Catalytic Ammonia Decomposition | ||||||
|---|---|---|---|---|---|---|---|---|
| Consumption Description | Specification | Consumption qty. | Consumption Description | Specification | Consumption qty. | Consumption Description | Specification | Consumption qty. |
| Natural gas process | CH4 >90% | 520 Nm3 | Refined methanol | GB338-92 first grade | 580 kg | Liquid ammonia | / | 600 kg |
| Catalyst and desulfurizer | / | 0.625 kg | Desalted water | Boiler water standard and Cl- < 3 ppm | 350 kg | Catalyst | / | 0.1 kg |
| Fuel gas | / | 480 Nm3 | Catalyst | / | 0.4 kg | Electricity | 380/220 V, 50 Hz | 1400 kWh |
| Low pressure vapor | / | 1.27 kg | Conduction oil | Max heating temperature: 320 °C | 0.35 kg | Circulating cooling water | Pressure: 0.3~0.4 MPa, Temp.: 27~32 °C | 75 t |
| Soft water | Boiler water standard | 1.67 t | Hydrogen | H2 ≥ 99.5% | 800 Nm3 | / | ||
| Electricity | 380/220 V, 50 Hz | 166 kWh | Nitrogen | O2 ≤ 0.2% | 400 Nm3 | |||
| Circulating cooling water | Pressure: 0.3~0.4 MPa, Temp.: 27~32 °C | 27 t | Electricity | 380/220 V, 50 Hz | 90 kWh | |||
| Instrument air | / | 42 Nm3 | Circulating cooling water | Pressure: 0.3~0.4 MPa, Temp.: 27~32 °C | 30 t | |||
| / | Fuel coal | / | 0.25 kg | |||||
| Instrument air (Nm3) | / | 200 | ||||||
References
- Liu, F.; Zhao, F.; Liu, Z.; Hao, H. The impact of fuel cell vehicle deployment on road transport greenhouse gas emissions: The China case. Int. J. Hydrogen Energy 2018, 43, 22604–22621. [Google Scholar] [CrossRef]
- Yamada, Y.; Ariyama, Y.; Ino, H. Energy consumption and CO2 emissions in fuel cell vehicles: Comparison of gasoline and natural gas. J. Jpn. Inst. Met. Mater. 2005, 69, 237–240. (In Japanese) [Google Scholar] [CrossRef]
- Bauer, C.; Hofer, J.; Althaus, H.J.; Del Duce, A.; Simons, A. The environmental performance of current and future passenger vehicles: Life Cycle Assessment based on a novel scenario analysis framework. Appl. Energy 2015, 157, 871–883. [Google Scholar] [CrossRef]
- Tong, F.; Jaramillo, P.; Azevedo, I.M.L. Comparison of Life Cycle Greenhouse Gases from Natural Gas Pathways for Light-Duty Vehicles. Energy Fuel 2015, 29, 6008–6018. [Google Scholar] [CrossRef]
- Hussain, M.M.; Dincer, I.; Li, X. A preliminary life cycle assessment of PEM fuel cell powered automobiles. Appl. Therm. Eng. 2007, 27, 2294–2299. [Google Scholar] [CrossRef]
- Granovskii, M.; Dincer, I.; Rosen, M.A. Life cycle assessment of hydrogen fuel cell and gasoline vehicles. Int. J. Hydrogen Energy 2006, 31, 337–352. [Google Scholar] [CrossRef]
- Lin, T.; Wu, Y.; He, X.; Zhang, S.; Hao, J. Well-to-Wheels Fossil Energy Consumption and CO2 Emissions of Hydrogen Fuel Cell Vehicles in China. Environ. Sci. 2018, 39, 3946–3953. (In Chinese) [Google Scholar]
- Xu, S.; Dong, C. Research on Energy Consumption and Emission of Vehicle in the Whole Life Cycle Based on GREET. Automob. Technol. Mat. 2014, 02, 10–13. (In Chinese) [Google Scholar]
- Bicer, Y.; Dincer, I. Comparative life cycle assessment of hydrogen, methanol and electric vehicles from well to wheel. Int. J. Hydrogen Energy 2017, 42, 3767–3777. [Google Scholar] [CrossRef]
- Offer, G.J.; Howey, D.; Contestabile, M.; Clague, R.; Brandon, N.P. Comparative analysis of battery electric, hydrogen fuel cell and hybrid vehicles in a future sustainable road transport system. Energy Policy 2010, 38, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Granovskii, M.; Dincer, I.; Rosen, M.A. Economic and environmental comparison of conventional, hybrid, electric and hydrogen fuel cell vehicles. J. Power Sources 2006, 159, 1186–1193. [Google Scholar] [CrossRef]
- Miotti, M.; Hofer, J.; Bauer, C. Integrated environmental and economic assessment of current and future fuel cell vehicles. Int. J. Life Cycle Assess. 2017, 22, 94–110. [Google Scholar] [CrossRef]
- Evangelisti, S.; Tagliaferri, C.; Brett, D.J.L.; Lettieri, P. Life cycle assessment of a polymer electrolyte membrane fuel cell system for passenger vehicles. J. Clean Prod. 2017, 142, 4339–4355. [Google Scholar] [CrossRef]
- Song, Y.; Wang, X.; Bi, S.; Wu, J.; Qiu, S. Assessment of fuel cell bus hydrogen energy system. Acta Energ. Sol. Sin. 2018, 39, 651–658. (In Chinese) [Google Scholar]
- Ahmadi, P.; Kjeang, E. Comparative life cycle assessment of hydrogen fuel cell passenger vehicles in different Canadian provinces. Int. J. Hydrogen Energy 2015, 40, 12905–12917. [Google Scholar] [CrossRef]
- Li, Q.; Yang, J.; Li, Q.; Zhang, L.; Wang, L. Life Cycle Assessment of Hydrogen Sources of Fuel Cell Vehicles. Res. Environ. Sci. 2003, 03, 59–61. (In Chinese) [Google Scholar]
- Zhu, H.; Yu, Z. Life Cycle Assessment of Hydrogen Pathways for Fuel Cell Vehicles. J. Tongji Univ. 2017, 45, 138–143, 151. (In Chinese) [Google Scholar] [CrossRef]
- Suleman, F.; Dincer, I.; Agelin-Chaab, M. Comparative impact assessment study of various hydrogen production methods in terms of emissions. Int. J. Hydrogen Energy 2016, 41, 8364–8375. [Google Scholar] [CrossRef]
- Hajjaji, N.; Pons, M.N.; Renaudin, V.; Houas, A. Comparative life cycle assessment of eight alternatives for hydrogen production from renewable and fossil feedstock. J. Clean Prod. 2013, 44, 177–189. [Google Scholar] [CrossRef]
- Yoo, E.; Kim, M.; Song, H.H. Well-to-wheel analysis of hydrogen fuel-cell electric vehicle in Korea. Int. J. Hydrogen Energy 2018, 43, 19267–19278. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, X. Well-to-wheels analysis of hydrogen based fuel-cell vehicle pathways in Shanghai. Energy 2006, 31, 471–489. [Google Scholar] [CrossRef]
- Hwang, J.J. Sustainability study of hydrogen pathways for fuel cell vehicle applications. Renew. Sustain. Energy Rev. 2013, 19, 220–229. [Google Scholar] [CrossRef]
- Kong, D.; Tang, W.; Liu, W.; Wang, M. Energy Consumption, Emissions and Economic Evaluation of Fuel Cell Vehicles. J. Tongji Univ. 2018, 46, 498–503, 523. (In Chinese) [Google Scholar]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life cycle assessment of hydrogen production via electrolysis—A review. J. Clean Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Zhao, G.; Pedersen, A.S. Life Cycle Assessment of Hydrogen Production and Consumption in an Isolated Territory. Procedia CIRP 2018, 69, 529–533. [Google Scholar] [CrossRef]
- Ally, J.; Pryor, T. Life-cycle assessment of diesel, natural gas and hydrogen fuel cell bus transportation systems. J. Power Sources 2007, 170, 401–411. [Google Scholar] [CrossRef] [Green Version]
- Simons, A.; Bauer, C. A life-cycle perspective on automotive fuel cells. Appl. Energy 2015, 157, 884–896. [Google Scholar] [CrossRef]
- Thomas, C.E. Fuel Cell and Battery Electric Vehicles Compared. Int. J. Hydrogen Energy 2009, 34, 6005–6020. [Google Scholar] [CrossRef]
- Hao, H.; Mu, Z.; Liu, Z.; Zhao, F. Abating transport GHG emissions by hydrogen fuel cell vehicles: Chances for the developing world. Front. Energy 2018, 12, 466–480. [Google Scholar] [CrossRef]
- Toyota Mirai Block Diagram: Detailed Fuel Cell Technology. Available online: https://wenku.baidu.com/view/16164da4a32d7375a41780cf.html (accessed on 5 January 2019). (In Chinese).
- Toyota Mirai Major Tables. Available online: https://toyota.jp/pages/contents/mirai/001_p_002/pdf/spec/mirai_spec_201801.pdf (accessed on 11 January 2019). (In Japanese).
- Lane, B.; Shaffer, B.; Samuelsen, G.S. Plug-in fuel cell electric vehicles: A California case study. Int. J. Hydrogen Energy 2017, 42, 14294–14300. [Google Scholar] [CrossRef]
- Universiteit Leiden. CML-IA Characterisation Factors. Available online: http://www.leidenuniv.nl/cml/ssp/databases/cmlia/cmlia.zip (accessed on 10 December 2018).
- Zackrisson, M.; Avellan, L.; Orlenius, J. Life cycle assessment of lithium-ion batteries for plug-in hybrid electric vehicles—Critical issues. J. Clean Prod. 2010, 18, 1519–1529. [Google Scholar] [CrossRef]
- Li, X. Research on Life Cycle Assessment of Scrapped Vehicle Based on GaBi Software. Master’s Thesis, Nankai University, Tianjin, China, 2011. (In Chinese). [Google Scholar]
- Li, J. Life Cycle Assessment and Analysis between Power System of Gas Car and Battery Electric Vehicles. Master’s Thesis, Hunan University, Changsha, China, 2014. [Google Scholar]
- Vehicle-IA. Available online: http://cdmd.cnki.com.cn/Article/CDMD-10532-1016309978.htm (accessed on 25 May 2019). (In Chinese).
- Chen, Y.; Yang, Y.; Li, X.; Dong, H.; Bai, R. Life Cycle Resource Consumption of Automotive Power Seats. Int. J. Environ. Stud. 2014, 71, 449–462. [Google Scholar] [CrossRef]
- Li, S. Life Cycle Assessment and Environmental Benefits Analysis of Electric Vehicles. Ph.D. Thesis, Jilin University, Changchun, China, 2014. [Google Scholar]
- Hu, X. Research on Performance Evaluation of Energy-saving and Emission Reduction of Plug-in Hybrid Electric Vehicle Based on Vehicle-IA and GaBi Platform. Bachelor’s Thesis, Chang’an University, Xi’an, China, 2017. [Google Scholar]
- Zhang, L.; Xu, G.; Zhang, W.; Zhang, C.; Wei, C. Life Cycle Assessment of Vice Dashboard Assembly of Automobile. J. Automot. Eng. 2015, 5, 263–269. (In Chinese) [Google Scholar]
- Yang, D.; Liu, J.; Yang, J.; Ding, N. Carbon footprint analysis of passenger car radial tires. China Popul. Resour. Environ. 2014, 24, 110–114. (In Chinese) [Google Scholar]
- Burnham, A.; Wang, M.; Wu, Y. Development and Application of GREET 2.7—The Transportation Vehicle-Cycle Model; Argonne National Laboratory: Chicago, IL, USA, 2006. [Google Scholar]
- Papasavva, S.; Kia, S.; Claya, J.; Gunther, R. Life Cycle Environmental Assessment of Paint Processes. J. Coat. Technol. 2002, 74, 65–76. [Google Scholar] [CrossRef]
- Yang, S. Ways to ensure successful drawing process for automobile panel. Die Mould Technol. 2014, 02, 44–48. (In Chinese) [Google Scholar]
- Hu, W. Study on the Characterization Method for Ni-MH Batteries Performance. Master’s Thesis, Tianjin University, Tianjin, China, 2006. [Google Scholar]
- Chen, S.; Chen, M. A Review on the Economy, Environmental Impact and Energy Efficiency Assessment for Clean-Energy Based on Life Cycle Analysis. J. Automot. Eng. 2008, 30, 465–469. (In Chinese) [Google Scholar]
- Electric Power Industry Statistics Compiled 2016. Available online: http://www.chinabookshop.net/electric-power-industry-statistics-compiled-2016-p-25839.html?osCsid=fchmcncjr6msrr7e746oplv421 (accessed on 25 May 2019). (In Chinese).
- Gao, Y.; Wang, J.; Zhu, Y.; Zhou, W. Research on Environmental Impact of Vehicle Power Battery Recycling. Automob. Parts 2014, 20, 41–43. (In Chinese) [Google Scholar]
- Cao, D.; Chu, C. Life Cycle Assessment and Its Application in Automotive Exhaust Emission Field. Automob. Technol. 2006, 2, 43–46. (In Chinese) [Google Scholar]
- Jiang, S.; Hao, H.; Chen, Y. Research on Carbon Emissions in Manufacturing Process of Lithium Ion Battery for Vehicles. In Proceedings of the 7th Annual Conference on Energy Economics and Management, Nanjing, China, 14–16 October 2016. [Google Scholar]
- Liu, Y. Comparison and Selection of Process of Hydrogen Production. Chem. Bioeng. 2007, 24, 72–74. (In Chinese) [Google Scholar]













| Main Parameters | Vehicle Mass | Battery Type | 0–100 km/h Acceleration | Max. Speed | Driving Range | Hydrogen Consumption per 100 km |
|---|---|---|---|---|---|---|
| Value | 1850 kg | Ni-MH battery | 9.6 s | 175 km/h | 486 km | 0.85 kg |
| Data sources | [32] | [31] | [31] | [32] | [31] | [31] |
| Vehicle body | Fuel cell | Energy storage battery | Hydrogen storage tank | Balancing device | Total mass |
| 1568.57 | 73.5 | 22.1 | 106.56 | 79.27 | 1850 |
| Fluid | |||||
| Lubricant | Brake fluid | Coolant | Wiper liquid | Additive | Total |
| 3.9 | 0.9 | 10.4 | 2.7 | 13.6 | 31.5 |
| Body | |||
|---|---|---|---|
| Mass (kg) | |||
| Body | 822.85 | ||
| Glass | 35 | ||
| Component | Energy consumption (electric power) (MJ/kg) | Component | Energy consumption (electric power) (MJ/kg) |
| Hood assembly | 1.47870 | Front side door assembly | 1.97488 |
| Hood external panel | 0.41143 | Front door external panel | 0.36142 |
| Hood internal panel | 0.41142 | Front door internal panel | 0.94754 |
| Hood welding | 0.65585 | Welding | 0.66592 |
| Top cover assembly | 1.19360 | Rear side door assembly | 1.97488 |
| Top cover | 0.35897 | Engine compartment assembly | 2.05793 |
| Front and rear cover beams | 0.75953 | Parts processing | 1.82428 |
| Top cover welding | 0.07509 | Welding | 0.23365 |
| Fender and lateral assembly | 5.07587 | Front battery bracket assembly | 1.00612 |
| Fender | 0.94252 | Rear battery bracket assembly | 1.00612 |
| L&R outer panel | 1.56238 | Cowl panel assembly | 1.14528 |
| Pillar A | 0.94756 | Back panel assembly | 1.14528 |
| Pillar B | 0.94754 | Floor assembly | 4.28929 |
| Welding | 0.67588 | Front floor assembly | 2.07985 |
| Trunk cover assembly | 3.17803 | Rear floor assembly | 2.07985 |
| Outer decklid | 1.34565 | Assembly | 0.12959 |
| Inner decklid | 1.10479 | Body assembly | 0.73594 |
| Welding | 0.72760 | / | / |
| Interior | Powertrain | Battery | Chassis | |||||||
| Power seat | 27.01 (kg) | Motor | 29 (kg) | Energy storage battery | 22.1 (kg) | Bracket | 29.9 (kg) | |||
| Dashboard | 3.7 (kg) | Controller | 21.5 (kg) | Fuel cell | 73.5 (kg) | Drive shaft | 84.25 (kg) | |||
| Energy consumption (kWh) | Energy consumption | Electric energy (MJ/kg) | Thermal energy (MJ/kg) | Energy consumption | Electricity (MJ/kg) | Thermal energy (MJ/kg) | Differential | 35.25 (kg) | ||
| Motor | / | Stator | 6.5647 | 2.0300 | Energy storage battery | 5.48 (kg) | 3.64 (kg) | Suspension system | 51.15 (kg) | |
| Skeleton | / | Armature winding | 5.3305 | 2.0300 | Fuel cell | Electricity (MJ/kg) | Thermal energy (MJ/kg) | Brake system | 48.45 (kg) | |
| Headrest | / | Iron core | 0.5464 | 0 | Proton exchange membrane | 1.15 | 0.71 | Wheels | 51.65 (kg) | |
| Slideway | / | Stator assembly | 0.6879 | 0 | Gas diffusion layer | 239.45 | 89.176 | Tires | 40.8 (kg) | |
| Angle adjuster | / | P-m rotor | 56.1509 | 6.7784 | Catalyst coating | 0.6007 | 0.0905 | Steering system | 32.55 (kg) | |
| Memory device | / | Motor shaft | 3.8387 | 2.7712 | Catalyst membrane | 512.5 | 0 | Electricity (MJ/kg) | Thermal energy (MJ/kg) | |
| Horizontal actuator | / | Electromotor shell | 4.1577 | 2.0881 | MEA assembly | 5.51 | 0 | |||
| Seat cushion assembly | / | assembly | 0.5167 | 0 | Bipolar plate | 22.2486 | 0 | Spring | 0.214229 | 2.771318 |
| Skin assembly | / | Energy consumption (kWh) | Energy consumption (kWh) | |||||||
| Decorative plastic | / | Wiring harness | / | Rims | 2.682571 | 0 | Assembly | 0.084027 | 0 | |
| Switch box | / | Total | 100 | Spokes | 1.258192 | 0 | Tires | 2.3495 | 0 | |
| Other parts | Mass (kg) | Electric energy (MJ/kg) | Other parts | Mass (kg) | Electric energy (MJ/kg) | Other parts | Mass (kg) | Electric energy (MJ/kg) |
| Decorative and blocking parts | 22.23 | 3 00 | Powertrain cooling system | 24.04 | 2.00 | Emission control element | 9.98 | 4.40 |
| Heating ventilation and air conditioning (HVAC) | 19.96 | 0 65 | Discharge system | 44.91 | 4.50 | Chassis electric system | 9.98 | 4.40 |
| Internal electric system | 9.98 | 4.40 | Power assembly electric system | 9.98 | 4.40 | / | / | / |
| Hydrogen storage tank | ||||||||
| Components | Electric energy (MJ/kg) | Thermal energy (MJ/kg) | ||||||
| Composite coating | 16.2 | 0 | ||||||
| Carbon fiber resin | 12.87 | 0 | ||||||
| Aluminum foil lining | 16.422 | 0 | ||||||
| Coating | Air Conditioning and Lighting | Heating | Material Handling | Welding | Workshop Compressed Air | |
|---|---|---|---|---|---|---|
| Energy | 2.72 | 2.18 | / | 0.45 | 0.61 | 0.9 |
| Thermal energy | / | / | 2.03 | / | / | / |
| Item | Specification | Consumption |
|---|---|---|
| Desalinated water (kg) | Suitable for boiler and Cl- < 3 ppm | 820 |
| Electricity (kWh) | 380/220 V, 50 Hz | 5500 |
| Tires (km) | Fluids (km) | Wiper Fluid (km) | Brake Fluid (km) | Coolant (km) | Battery (Number of Cycles) | |
|---|---|---|---|---|---|---|
| Service duration | 62,500 | 6250 | 12,500 | 62,500 | 62,500 | 2000 |
| Replacement times | 4 | 39 | 24 | 4 | 4 | 3 |
| Methanol Steam Reforming | Steam Methane Reforming (SMR) | Catalytic Ammonia Decomposition | Electrolysis | |
|---|---|---|---|---|
| ADP (e) (kg Sb-Eq) | 2.44E-4 | 1.73E-3 | 3.07E-3 | 0.0158 |
| ADP (f) (MJ) | 2.79E+4 | 3.85E+5 | 3.52E+5 | 1.24E+6 |
| GWP (kg CO2-Eq) | 2.78E+3 | 4.58E+3 | 3.66E+4 | 1.24E+5 |
| AP (kg SO2-Eq) | 11.7 | 12.3 | 175 | 525 |
| EP (kg phosphate-Eq) | 0.876 | 1.02 | 17.3 | 36.3 |
| POCP (kg ethene-Eq) | 15.3 | 1.64 | 16.6 | 50 |
| ODP (kg R11-Eq) | 5.79E-10 | 3.32E-10 | 7.29E-9 | 4.06E-8 |
| Current | Scenario 1 | Scenario 2 | Scenario 3 | |
|---|---|---|---|---|
| Thermal power | 71.60 | 61.60 | 51.60 | 41.60 |
| Hydropower | 19.71 | 19.71 | 19.71 | 19.71 |
| Nuclear power | 3.56 | 3.56 | 3.56 | 3.56 |
| Wind power | 4.02 | 9.02 | 14.02 | 19.02 |
| Solar power | 1.11 | 6.11 | 11.11 | 16.11 |
| Total | 100 | 100 | 100 | 100 |
| Electrolysis (Wind Power) | Electrolysis (Hydropower) | Electrolysis (Solar Power) | Electrolysis (Nuclear Power) | |
|---|---|---|---|---|
| GWP | 2.08E+3 | 1.24E+3 | 1.06E+4 | 789 |
| AP | 6.32 | 0.759 | 42.5 | 6.18 |
| EP | 0.684 | 0.113 | 3.42 | 0.795 |
| POCP | 0.256 | 0.0455 | 3.86 | 0.454 |
| ODP | 3.04E-9 | 1.02E-10 | 5.75E-8 | 1.1E-6 |
| Comprehensive value | 2.01E-11 | 9.01E-12 | 1.23E-10 | 1.25E-10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Hu, X.; Liu, J. Life Cycle Assessment of Fuel Cell Vehicles Considering the Detailed Vehicle Components: Comparison and Scenario Analysis in China Based on Different Hydrogen Production Schemes. Energies 2019, 12, 3031. https://doi.org/10.3390/en12153031
Chen Y, Hu X, Liu J. Life Cycle Assessment of Fuel Cell Vehicles Considering the Detailed Vehicle Components: Comparison and Scenario Analysis in China Based on Different Hydrogen Production Schemes. Energies. 2019; 12(15):3031. https://doi.org/10.3390/en12153031
Chicago/Turabian StyleChen, Yisong, Xu Hu, and Jiahui Liu. 2019. "Life Cycle Assessment of Fuel Cell Vehicles Considering the Detailed Vehicle Components: Comparison and Scenario Analysis in China Based on Different Hydrogen Production Schemes" Energies 12, no. 15: 3031. https://doi.org/10.3390/en12153031