Neuroendocrine Tumors: Clinical, Histological and Immunohistochemical Perspectives and Case Report—Mature Teratoma in a 16-Year-Old Girl
Abstract
:1. Introduction
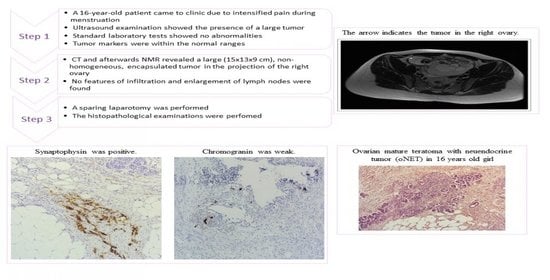
2. Case Presentation
3. Methods
IHC
4. Results
4.1. Histopathological Findings
4.1.1. TERATOMA
4.1.2. NET
4.2. Immunohistochemical Findings of NET
4.3. Immunohistochemical Findings of Teratoma
5. Discussion
NET
| Year | Author | Diagnosis | Age |
|---|---|---|---|
| 2020 | Maccora et al. [38] | mature teratoma with insular carcinoid tumor of the ovary | 68 |
| 2019 | Chai et al. [39] | strumal carcinoid tumor of the ovary | 63 |
| 2019 | Yan et al. [40] | multiple endocrine neoplasia type 1-related atypical ovarian carcinoid | 30 |
| 2019 | Borghese et al. [41] | bilateral MCT with foci of ovarian strumal carcinoid, developed lymph node para aortic metastasis after 30 years from primary diagnosis | ? |
| 2019 | Hsu et al. [42] | primary ovarian mucinous carcinoid tumor, atypical type, very aggressive | 33 |
| 2018 | Ishida et al. [43] | stromal carcinoid of the ovary | 68 |
| 2018 | Macháleková et al. [44] | stromal carcinoid of the ovary | 46, 52 |
| 2018 | Niu et al. [45] | carcinoid arising from the teratomatous bronchial mucosa in an ovarian MCT | 22 |
| 2017 | Fiore et al. [46] | goblet-cell carcinoid of the ovary | 18 |
| 2016 | Kim [47] | carcinoid tumor of the trabecular type arising from an MCT in ovary | 25 |
| 2016 | Erdenebaatar et al. [48] | insular carcinoid tumor of the ovary with a trabecular component | 70 |
| 2016 | Kim et al. [49] | primary ovarian mixed strumal and mucinous carcinoid arising in an ovarian MCT | 39 |
| 2016 | Vora et al. [17] | well-differentiated carcinoid tumor with no surface epithelial involvement, and a mature teratoma in the contralateral ovary, a mature teratoma; strumal carcinoid within the ovarian parenchyma; poorly differentiated carcinoma with neuroendocrine differentiation | 40, 26, 63, 32 |
| 2015 | Kim et al. [50] | primary ovarian carcinoid tumor with loss of neuroendocrine growth pattern, increased mitotic activity and large areas of coagulative tumor necrosis, atypical carcinoid | 21 |
| 2015 | Mieczkowska et al. [51] | primary ovarian carcinoid in mature teratoma of one ovary, co-existing with primary epithelial carcinoma of another ovary | |
| 2015 | Târcoveanu et al. [52] | ovarian strumal carcinoid and cystic lymphangioma | 55 |
| 2015 | Muller et al. [53] | ovarian strumal carcinoid (peptide YY producing) | 34 |
| 2014 | Goldman et al. [54] | secondary to carcinoid heart disease caused by a primary ovarian carcinoid tumor | 61 |
| 2014 | Gupta et al. [55] | primary ovarian carcinoid tumor simulating virilizing tumor of the ovary | 62 |
| 2014 | Kumar et al. [56] | carcinoid of the ovary | 53 |
| 2013 | Sulaiman et al. [57] | strumal carcinoid tumor stage 1A of the ovary | 30 |
| 2012 | Takatori et al. [58] | strumal carcinoid of the ovary (peptide YY producing) | 48 |
| 2013 | Hayashi et al. [59] | primary strumal carcinoid tumor of the ovary | 45 |
| 2011 | Takeuchi et al. [60] | strumal carcinoid tumor of the ovary primary strumal carcinoid tumor of the ovary | 72 77 |
| 2011 | Matsunami et al. [61] | strumal carcinoid tumor of the ovary (peptide YY producing) | 45 |
| 2010 | Marcy et al. [62] | lethal, malignant, metastatic struma ovarii | 45 |
| 2010 | Bai et al. [63] | primary ovarian trabecular carcinoid tumor | 55 |
| 2010 | Kurabayashi et al. [64] | primary strumal carcinoid tumor of the ovary with multiple bone and breast metastases | 34 |
| 2009 | Suneja et al. [65] | primary malignant melanoma in cystic teratoma of ovary | 50 |
| 2009 | Guney et al. [66] | primary carcinoid tumor arising in a mature cystic teratoma | 54 |
| 2009 | Gungor et al. [67] | primary ovarian carcinoid arising from a mature cystic teratoma | 47 |
| 2008 | Lagoudianakis et al. [68] | primary ovarian insular carcinoid tumor | 44 |
| 2008 | Gorin & Sastre-Garau [69] | strumal carcinoid tumor of the ovary | 63 |
| 2007 | Somak et al. [70] | primary carcinoid tumor of the ovary | 55 |
| 2007 | Morken et al. [71] | primary ovarian carcinoid tumor | 70 |
| 2006 | Chatzipantelis et al. [72] | insular carcinoid and mucinous cystadenoma of low malignant potential, arising in a cystic teratoma | 57 |
| 2006 | Karavolos et al. [73] | primary mucinous carcinoid tumor of the ovary | 34 |
| 2005 | Kopf et al. [74] | primary carcinoid tumor of the ovary | 79 |
| 2003 | Kuscu et al. [75] | ovarian carcinoid stage IA | 47 |
| 2002 | Matsuda et al. [76] | strumal carcinoid tumor of the ovary (peptide YY producing) | 50 |
| 2000 | McMurray [77] | benign left ovarian cystic teratoma, and a right carcinoid tumor of the ovary | 57 |
| 1996 | Kasantikul et al. [78] | primary ovarian carcinoid (insular, trabecular and mucinous components) | 53 |
| 1996 | Chou et al. [79] | primary ovarian carcinoid tumor | 25 |
| 1995 | Takemori et al. [80] | ovarian strumal carcinoid in association with dermoid cyst and mucinous cystadenoma in the same ovary | 54 |
| 1995 | Yaegashi et al. [81] | primary trabecular carcinoid of the ovary | 43 |
| 1993 | Kataoka et al. [82] | trabecular carcinoid tumor associated intimately with thyroid follicle-like structures, strumal carcinoid arising in a benign cystic teratoma | 41 |
| 1992 | Erhan et al. [83] | primary carcinoid tumor | 55 |
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikolic, A.; Volarevic, V.; Armstrong, L.; Lako, M.; Stojkovic, M. Primordial Germ Cells: Current Knowledge and Perspectives. Stem Cells Int. 2016, 2016, 1741072. [Google Scholar] [CrossRef] [Green Version]
- Kolasa, A.; Misiakiewicz, K.; Marchlewicz, M.; Wiszniewska, B. The generation of spermatogonial stem cells and spermatogonia in mammals. Reprod. Biol. 2012, 12, 5–23. [Google Scholar] [CrossRef]
- Pierce, J.L.; Frazier, A.L.; Amatruda, J.F. Pediatric Germ Cell Tumors: A Developmental Perspective. Adv. Urol. 2018, 2018, 9059382. [Google Scholar] [CrossRef] [Green Version]
- Stella, F.; Davoli, F. Giant mediastinal mature teratoma with increased exocrine pancreatic activity presenting in a young woman: A case report. J. Med. Case Rep. 2011, 5, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaaban, A.M.; Rezvani, M.; Elsayes, K.M.; Baskin, H., Jr.; Mourad, A.; Foster, B.R.; Jarboe, E.A.; Menias, C.O. Ovarian malignant germ cell tumors: Cellular classification and clinical and imaging features. Radiographics 2014, 34, 777–801. [Google Scholar] [CrossRef]
- Shanthi, E.; Parimala, A.; Chander, R.V.; Jayashree, K.; Sulochana, S. Ovarian struma—Report of a rare case. J. Clin. Diagn. Res. 2015, 9, QJ01. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; Sharma, S.; Agarwal, S. Malignant transformation in mature cystic teratoma of the ovary: A retrospective study of eight cases and review of literature. Menop. Rev. 2018, 17, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, H.; Balkawade, N.; Gore, C.; Deshpande, A. Struma ovarii: A rare case report. Int. J. Pharm. Biomed. Sci. 2012, 3, 152–154. [Google Scholar]
- Chauhan, A.; Yu, Q.; Ray, N.; Farooqui, Z.; Huang, B.; Durbin, E.B.; Tucker, T.; Evers, M.; Arnold, S.; Anthony, L.B. Global burden of neuroendocrine tumors and changing incidence in Kentucky. Oncotarget 2018, 9, 19245–19254. [Google Scholar] [CrossRef] [Green Version]
- Kulke, M.H.; Mayer, R.J. Carcinoid tumors. N. Engl. J. Med. 1999, 340, 858–868. [Google Scholar] [CrossRef]
- Estrozi, B.; Bacchi, C.E. Neuroendocrine tumors involving the gastroenteropancreatic tract: A clinicopathological evaluation of 773 cases. Clinics 2011, 66, 1671–1675. [Google Scholar]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef]
- Rindi, G.; Petrone, G.; Inzani, F. The 2010 WHO classification of digestive neuroendocrine neoplasms: A critical appraisal four years after its introduction. Endocr. Pathol. 2014, 25, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Klimstra, D.S. Pathologic Classification of Neuroendocrine Neoplasms. Hematol. Clin. N. Am. 2016, 30, 1–19. [Google Scholar] [CrossRef]
- Rouzbahman, M.; Clarke, B. Neuroendocrine tumors of the gynecologic tract: Select topics. Semin. Diagn. Pathol. 2013, 30, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Gardner, G.J.; Reidy-Lagunes, D.; Gehrig, P.A. Neuroendocrine tumors of the gynecologic tract: A Society of Gynecologic Oncology (SGO) clinical document. Gynecol. Oncol. 2011, 122, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Vora, M.; Lacour, R.A.; Black, D.R.; Turbat-Herrera, E.A.; Gu, X. Neuroendocrine tumors in the ovary: Histogenesis, pathologic differentiation, and clinical presentation. Arch. Gynecol. Obstet. 2016, 293, 659–665. [Google Scholar] [CrossRef]
- Metwally, I.H.; Elalfy, A.F.; Awny, S.; Elzahaby, I.A.; Abdelghani, R.M. Primary ovarian carcinoid: A report of two cases and a decade registry. J. Egypt. Natl. Cancer Inst. 2016, 28, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soga, J.; Osaka, M.; Yakuwa, Y. Carcinoids of the ovary: An analysis of 329 reported cases. J. Exp. Clin. Cancer Res. 2000, 19, 271–280. [Google Scholar]
- Feldman, J.M.; Lee, E.M. Serotonin content of foods: Effect on urinary excretion of 5-hydroxyindoleacetic acid. Am. J. Clin. Nutr. 1985, 42, 639–643. [Google Scholar] [CrossRef] [Green Version]
- Oleinikov, K.; Avniel-Polak, S.; Gross, D.J.; Grozinsky-Glasberg, S. Carcinoid Syndrome: Updates and Review of Current Therapy. Curr. Treat. Options Oncol. 2019, 20, 70. [Google Scholar] [CrossRef]
- Davis, Z.; Moertel, C.G.; McIlrath, D.C. The malignant carcinoid syndrome. Surg. Gynecol. Obstet. 1973, 137, 637–644. [Google Scholar]
- Royal College of Obstetricians and Gynaecologists. Management of suspected ovarian masses in premenopausal women. In Green-Top Guideline; RCOG: London, UK, 2011. [Google Scholar]
- Spaczyński, M.; Bidziński, M.; Basta, A.; Dańska-Bidzińska, A.; Breborowicz, G.H.; Emerich, J.; Grabiec, M.; Kedzia, W.; Kornafel, J.; Kotarski, J.; et al. Polish Gynecological Society’s recommendations regarding ovarian cancer. Ginekol. Pol. 2006, 77, 495–501. [Google Scholar]
- Chen, R.F.; Li, J.; Zhu, T.T.; Yu, H.L.; Lu, X. Fertility-sparing surgery for young patients with borderline ovarian tumors (BOTs): Single institution experience. J. Ovarian Res. 2016, 9, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renaud, E.J.; Sømme, S.; Islam, S.; Cameron, D.B.; Gates, R.L.; Williams, R.F.; Jancelewicz, T.; Oyetunji, T.A.; Grabowski, J.; Diefenbach, K.A.; et al. Ovarian masses in the child and adolescent: An American Pediatric Surgical Association Outcomes and Evidence-Based Practice Committee systematic review. J. Pediatr. Surg. 2019, 54, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Kos-Kudła, B.; Blicharz-Dorniak, J.; Strzelczyk, J.; Bałdys-Waligórska, A.; Bednarczuk, T.; Bolanowski, M.; Boratyn-Nowicka, A.; Borowska, M.; Cichocki, A.; Ćwikła, J.B.; et al. Diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2017, 68, 79–110. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hongo-Kohama, M.; Kurata, A.; Hashimoto, H.; Fujita, K.; Horiuchi, H.; Nagao, T.; Kuroda, M. Vascular Smooth Muscle Cell Maturation Stage and Ki-67 Index are Diagnostic Biomarkers for Pathologic Grade of Ovarian Teratoma. Int. J. Gynecol. Pathol. 2017, 36, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Wreesmann, V.B.; Nixon, I.J.; Rivera, M.; Katabi, N.; Palmer, F.; Ganly, I.; Shaha, A.R.; Tuttle, R.M.; Shah, J.P.; Patel, S.G.; et al. Prognostic value of vascular invasion in well-differentiated papillary thyroid carcinoma. Thyroid 2015, 25, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Cappello, F.; Barbato, F.; Tomasino, R.M. Mature teratoma of the uterine corpus with thyroid differentiation. Pathol. Int. 2000, 50, 546–548. [Google Scholar] [CrossRef]
- Ernst, J.; Ikenberg, K.; Apel, B.; Schumann, D.M.; Huber, G.; Studer, G.; Rordorf, T.; Riesterer, O.; Rössle, M.; Korol, D.; et al. Expression of CK19 is an independent predictor of negative outcome for patients with squamous cell carcinoma of the tongue. Oncotarget 2016, 7, 76151–76158. [Google Scholar] [CrossRef] [PubMed]
- Abouhashem, N.S.; Talaat, S.M. Diagnostic utility of CK19 and CD56 in the differentiation of thyroid papillary carcinoma from its mimics. Pathol. Res. Pract. 2017, 213, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.H.; Yaar, M.; Bhawan, J. Cutaneous immunoreactivity of D2-40 antibody beyond the lymphatics. Am. J. Dermatopathol. 2007, 29, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.L.; Jolliffe, E.; Hertweck, S.P. Ovarian Teratoma Associated with Coexisting Anti-N-Methyl-D-Aspartate Receptor and Glial Fibrillary Acidic Protein Autoimmune Meningoencephalitis in an Adolescent Girl: A Case Report. J. Pediatr. Adolesc. Gynecol. 2018, 31, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jones, A.; Jenkins, S.M.; Huang, Y. Ki67 Proliferative Index in Carcinoid Tumors Involving Ovary. Endocr. Pathol. 2018, 29, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray-Coquard, I.; Morice, P.; Lorusso, D.; Prat, J.; Oaknin, A.; Pautier, P.; Colombo, N. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv1–iv18. [Google Scholar] [CrossRef]
- Maccora, D.; Caldarella, C.; Leombroni, M.; De Stefano, V.; Leccisotti, L. Incidental Finding of an Ovarian Carcinoid on 11C-Methionine PET/CT. Clin. Nucl. Med. 2020, 45, e483–e485. [Google Scholar] [CrossRef]
- Chai, W.; Zhang, W.; Zhou, L.; Sun, X.; Jia, G. Strumal carcinoid tumor of the ovary: A rare case report. Medicine 2019, 98, e18009. [Google Scholar] [CrossRef]
- Yan, B.; Wang, Y.; Zhang, Y.; Lou, W. Teratoma-associated anti-N-methyl-D-aspartate receptor encephalitis: A case report and literature review. Medicine 2019, 98, e15765. [Google Scholar] [CrossRef]
- Borghese, M.; Razzore, P.; Ferrero, A.; Daniele, L.; Mariani, L.L.; Sgro, L.G.; De Rosa, G.; Biglia, N. Metastatic Bilateral Strumal Carcinoid: A Case Report and Review of the Literature. Anticancer Res. 2019, 39, 5053–5056. [Google Scholar] [CrossRef]
- Hsu, W.W.; Mao, T.L.; Chen, C.H. Primary ovarian mucinous carcinoid tumor: A case report and review of literature. Taiwan. J. Obstet. Gynecol. 2019, 58, 570–573. [Google Scholar] [CrossRef]
- Ishida, M.; Arimoto, T.; Sandoh, K.; Okano, K.; Ebisu, Y.; Ito, H.; Matsumoto, M.; Mizokami, T.; Kita, M.; Okada, H.; et al. Imprint cytology of strumal carcinoid of the ovary: A case report with immunocytochemical analysis. Diagn. Cytopathol. 2019, 47, 218–221. [Google Scholar] [CrossRef]
- Macháleková, K.; Kolníková, G.; Redecha, M.; Žúbor, P.; Kajo, K. Strumal carcinoid of the ovary—Report of two cases and review of literature. Ceska Gynekol. 2018, 83, 452–457. [Google Scholar] [PubMed]
- Niu, D.; Li, Z.; Sun, L.; Cao, D. Carcinoid Arising From the Teratomatous Bronchial Mucosa in a Mature Cystic Teratoma of the Ovary: A Case Report. Int. J. Gynecol. Pathol. 2018, 37, 123–127. [Google Scholar] [CrossRef]
- Fiore, M.G.; Rossi, R.; Covelli, C.; Loizzi, V.; Piscitelli, D.; Cormio, G. Goblet-cell carcinoid of the ovary: A case report with ultrastructural analysis. J. Obstet. Gynaecol. 2017, 37, 266–267. [Google Scholar] [CrossRef]
- Kim, S.A.; Kwon, Y.; Kim, S.; Joung, H. Assessment of Dietary Mercury Intake and Blood Mercury Levels in the Korean Population: Results from the Korean National Environmental Health Survey 2012–2014. Int. J. Environ. Res. Public Health 2016, 13, 877. [Google Scholar] [CrossRef] [Green Version]
- Erdenebaatar, C.; Yamaguchi, M.; Saito, F.; Motooka, C.; Tashiro, H.; Katabuchi, H. An Ovarian Carcinoid Tumor with Peptide YY-Positive Insular Component: A Case Report and Review of the Literature. Int. J. Gynecol. Pathol. 2016, 35, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.R.; Ha, S.Y.; Shin, J.W.; Lim, S.; Park, C.Y.; Cho, H.Y. Primary ovarian mixed strumal and mucinous carcinoid arising in an ovarian mature cystic teratoma. J. Obstet. Gynaecol. Res. 2016, 42, 211–216. [Google Scholar] [CrossRef]
- Kim, H.S.; Yoon, G.; Jang, H.I.; Song, S.Y.; Kim, B.G. Primary ovarian carcinoid tumor showing unusual histology and nuclear accumulation of β-catenin. Int. J. Clin. Exp. Pathol. 2015, 8, 5749–5752. [Google Scholar] [PubMed]
- Mieczkowska, E.; Marciniak, A.; Szydłowska, I.; Brodowska, A.; Starczewski, A. Rare case of coexistence of primary ovarian carcinoid in mature teratoma with primary serous carcinoma in second ovary—A case report. Eur. J. Gynaecol. Oncol. 2015, 36, 330–332. [Google Scholar]
- Târcoveanu, E.; Vasilescu, A.; Fotea, V.; Ciobanu, D.; Crumpei, F.; Bradea, C. Rare Tumors, Rare Association: Ovarian Strumal Carcinoid—Retroperitoneal Cystic Lymphangioma. Chirurgia 2015, 110, 294–299. [Google Scholar]
- Muller, K.E.; Tafe, L.J.; Gonzalez, J.L.; West, L.A.; Schned, A.R. Ovarian strumal carcinoid producing peptide YY associated with severe constipation: A case report and review of the literature. Int. J. Gynecol. Pathol. 2015, 34, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Goldman, T.; Adamson, K.; Yang, E. Resolution of right-sided heart failure symptoms after resection of a primary ovarian carcinoid tumor. Tex. Heart Inst. J. 2014, 41, 533–536. [Google Scholar] [CrossRef]
- Gupta, B.; Suneja, A.; Vaid, N.B.; Bhatia, A. Primary ovarian carcinoid tumor simulating virilizing tumor of the ovary: A rare entity. Indian J. Cancer 2014, 51, 529. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Rajwanshi, A.; Dey, P. Carcinoid of the ovary: Diagnostic challenge on fine-needle aspiration cytology. Diagn. Cytopathol. 2014, 42, 612–614. [Google Scholar] [CrossRef]
- Sulaiman, S.; Chia, Y.N.; Namuduri, R.V. Strumal carcinoid tumour of the ovary presenting with severe constipation. Singapore Med. J. 2013, 54, e21–e23. [Google Scholar] [PubMed] [Green Version]
- Takatori, E.; Shoji, T.; Miura, J.; Takeuchi, S.; Yoshizaki, A.; Sugiyama, T. Case of peptide-YY-producing strumal carcinoid of the ovary: A case report and review. J. Obstet. Gynaecol. Res. 2012, 38, 1266–1270. [Google Scholar] [CrossRef]
- Hayashi, T.; Haba, R.; Kushida, Y.; Kadota, K.; Katsuki, N.; Miyai, Y.; Shibuya, S.; Sasaki, M.; Bando, K.; Matsunaga, T.; et al. Cytopathologic characteristics of the primary strumal carcinoid tumor of the ovary: A case report with emphasis on differential diagnostic considerations. Diagn. Cytopathol. 2013, 41, 812–816. [Google Scholar] [CrossRef]
- Takeuchi, M.; Matsuzaki, K.; Uehara, H. Primary carcinoid tumor of the ovary: MR imaging characteristics with pathologic correlation. Magn. Reson. Med. Sci. 2011, 10, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Matsunami, K.; Takagi, H.; Ichigo, S.; Murase, T.; Ikeda, T.; Imai, A. Peptide YY producing strumal carcinoid tumor of the ovary. Eur. J. Gynaecol. Oncol. 2011, 32, 201–202. [Google Scholar]
- Marcy, P.Y.; Thariat, J.; Benisvy, D.; Azuar, P. Lethal, malignant, metastatic struma ovarii. Thyroid 2010, 20, 1037–1040. [Google Scholar] [CrossRef]
- Bai, X.; Li, N.; Wang, F.; Li, S.; Yu, Q. Primary ovarian trabecular carcinoid tumor: A case report and literature review. Arch. Gynecol. Obstet. 2010, 282, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Kurabayashi, T.; Minamikawa, T.; Nishijima, S.; Tsuneki, I.; Tamura, M.; Yanase, T.; Hashidate, H.; Shibuya, H.; Motoyama, T. Primary strumal carcinoid tumor of the ovary with multiple bone and breast metastases. J. Obstet. Gynaecol. Res. 2010, 36, 567–571. [Google Scholar] [CrossRef]
- Suneja, A.; Yadav, P.; Sharma, A.; Vaid, N.B.; Singh, B.; Grover, R.K. Primary malignant melanoma in cystic teratoma of ovary. Indian J. Cancer 2009, 46, 340. [Google Scholar] [CrossRef] [PubMed]
- Guney, N.; Sayilgan, T.; Derin, D.; Ozcan, D. Primary carcinoid tumor arising in a mature cystic teratoma of the ovary: A case report. Eur. J. Gynaecol. Oncol. 2009, 30, 223–225. [Google Scholar] [PubMed]
- Gungor, T.; Altinkaya, O.; Ozat, M.; Sirvan, L.; Yalcin, H.; Mollamahmutoglu, L. Primary adenocarcinoid tumor of the ovary arising in mature cystic teratoma. A case report. Eur. J. Gynaecol. Oncol. 2009, 30, 110–112. [Google Scholar]
- Lagoudianakis, E.E.; Markogiannakis, H.; Karantzikos, G.; Papadima, A.; Alevizos, L.; Manouras, A. Primary insular carcinoid of the ovary. Eur. J. Gynaecol. Oncol. 2008, 29, 554–555. [Google Scholar] [PubMed]
- Gorin, I.; Sastre-Garau, X. Strumal carcinoid tumor of the ovary. J. Clin. Oncol. 2008, 26, 2780–2781. [Google Scholar] [CrossRef] [PubMed]
- Somak, R.; Shramana, M.; Vijay, S.; Nita, K. Primary carcinoid tumor of the ovary: A case report. Arch. Gynecol. Obstet. 2008, 277, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Morken, N.H.; Majak, B.; Kahn, J.A. Insulin producing primary ovarian carcinoid tumor. Acta Obstet. Gynecol. Scand. 2007, 86, 500–501. [Google Scholar] [CrossRef]
- Chatzipantelis, P.; Mavrogiorgis, A.; Kairi-Vassilatou, E.; Pafiti, A. Ovarian neoplasm composed of an insular carcinoid tumor and a borderline mucinous cystadenoma arising in a mature cystic teratoma: A case report. Eur. J. Gynaecol. Oncol. 2006, 27, 636–637. [Google Scholar] [PubMed]
- Karavolos, S.; Caplin, M.; Benjamin, E.; Crow, J.; Mould, T. Primary mucinous carcinoid tumour of the ovary: A case report. Eur. J. Gynaecol. Oncol. 2006, 27, 618–620. [Google Scholar] [PubMed]
- Kopf, B.; Rosti, G.; Lanzanova, G.; Marangolo, M. Locally advanced ovarian carcinoid. J. Exp. Clin. Cancer Res. 2005, 24, 313–316. [Google Scholar] [PubMed]
- Kuscu, E.; Eroglu, D.; Ozdemir, B.H.; Secme, S.; Haberal, A. Primary carcinoid tumor of the ovary: A case report. Eur. J. Gynaecol. Oncol. 2003, 24, 574–576. [Google Scholar]
- Matsuda, K.; Maehama, T.; Kanazawa, K. Strumal carcinoid tumor of the ovary: A case exhibiting severe constipation associated with PYY. Gynecol. Oncol. 2002, 87, 143–145. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.E. A case of diarrhea and orthopnea in a 57-year-old female. WMJ 2000, 99, 25–28, 34. [Google Scholar]
- Kasantikul, V.; Triratanachart, S.; Maneesri, S.; Panichabhongse, V. Primary ovarian carcinoid: A mixture of insular, trabecular and mucinous components. J. Med. Assoc. Thai. 1996, 79, 200–204. [Google Scholar]
- Chou, Y.Y.; Shun, C.T.; Huang, S.C.; Chuang, S.M. Primary ovarian carcinoid tumor. J. Formos. Med. Assoc. 1996, 95, 148–152. [Google Scholar]
- Takemori, M.; Nishimura, R.; Sugimura, K.; Obayashi, C.; Yasuda, D. Ovarian strumal carcinoid with markedly high serum levels of tumor markers. Gynecol. Oncol. 1995, 58, 266–269. [Google Scholar] [CrossRef]
- Yaegashi, N.; Tsuiki, A.; Shimizu, T.; Kobayashi, N.; Sato, S.; Namiki, T.; Motoyama, T.; Katayama, Y.; Yajima, A. Ovarian carcinoid with severe constipation due to peptide YY production. Gynecol. Oncol. 1995, 56, 302–306. [Google Scholar] [CrossRef]
- Kataoka, A.; Nishida, T.; Sugiyama, T.; Ohta, S.; Tomita, J.; Yakushiji, M. Strumal carcinoid of the ovary. Kurume Med. J. 1993, 40, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Erhan, Y.; Dikicioğlu, E.; Alkanat, M.B.; Ozdemir, N. Primary carcinoid tumor of the ovary—A case report. Acta Oncol. 1992, 31, 790–792. [Google Scholar] [CrossRef] [PubMed]





| Symptoms of Carcinoid Syndrome | Absent | |
|---|---|---|
| Tumor marker concentrations | Chromogranin A | 28.21 ug/L (normal: 0–100 ug/L) |
| ** 5-HIAA—mg/24 h | 18.85 mg/24 h (normal: 2–6 mg/24 h) | |
| Pelvic computed tomography (CT) | A 2.0 × 3.5 cm mass adjacent to the posterior wall of the uterus, slightly strengthening after the administration of contrast (postoperative lesions and the left part of the ovary) | |
| Tc99 receptor scintigraphy | No somatostatin receptor expression | |
| * PET/CT GAL68 | Lack of somatostatin receptor expression in the operated area. A small focal point with increased receptor expression in pineal topography | |
| Magnetic resonance imaging (MRI) | Pineal topography showed an oval structure measuring 11 × 10 × 9 mm with features of a cyst | |
| Pelvic ultrasonography (USG) of the pelvis minor | Vague picture in the right appendage projection | |
| MRI of the pelvis minor | No lesions suspected of being cancerous were found | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowińska-Przepiera, E.; Starzyński, D.; Syrenicz, A.; Dziuba, I.; Wiszniewska, B.; Rzeszotek, S. Neuroendocrine Tumors: Clinical, Histological and Immunohistochemical Perspectives and Case Report—Mature Teratoma in a 16-Year-Old Girl. Pathophysiology 2021, 28, 373-386. https://doi.org/10.3390/pathophysiology28030025
Sowińska-Przepiera E, Starzyński D, Syrenicz A, Dziuba I, Wiszniewska B, Rzeszotek S. Neuroendocrine Tumors: Clinical, Histological and Immunohistochemical Perspectives and Case Report—Mature Teratoma in a 16-Year-Old Girl. Pathophysiology. 2021; 28(3):373-386. https://doi.org/10.3390/pathophysiology28030025
Chicago/Turabian StyleSowińska-Przepiera, Elżbieta, Dariusz Starzyński, Anhelli Syrenicz, Ireneusz Dziuba, Barbara Wiszniewska, and Sylwia Rzeszotek. 2021. "Neuroendocrine Tumors: Clinical, Histological and Immunohistochemical Perspectives and Case Report—Mature Teratoma in a 16-Year-Old Girl" Pathophysiology 28, no. 3: 373-386. https://doi.org/10.3390/pathophysiology28030025