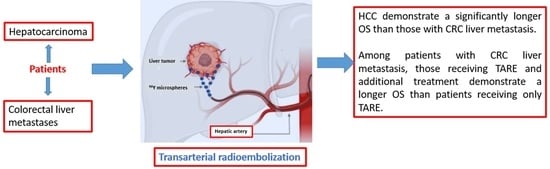
The Influence of Additional Treatments on the Survival of Patients Undergoing Transarterial Radioembolization (TARE)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Pre-Treatment Imaging
2.3. The 90Y-TARE Procedure
2.4. Post-Treatment Imaging
2.5. Statistics
3. Results
3.1. Patients with HCC
3.2. Patients with CRC Liver Metastases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mikell, J.K.; Dewaraja, Y.K.; Owen, D. Transarterial radioembolization for hepatocellular carcinoma and hepatic metastases: Clinical aspects and dosimetry models. Semin. Radiat. Oncol. 2020, 30, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef]
- Casáns-Tormo, I.; Guijarro-Rosaleny, J.; Lluch-García, P.; Rodríguez-Parra, H.; Roselló-Keränen, S.; Asensio-Valero, L. Evaluation of results after 112 radioembolizations with 90y-microspheres. Rev. Española De Med. Nucl. E Imagen Mol. 2023, 42, 255–264. [Google Scholar] [CrossRef]
- Emmons, E.C.; Bishay, S.; Du, L.; Krebs, H.; Gandhi, R.T.; Collins, Z.S.; O’Hara, R.; Akhter, N.M.; Wang, E.A.; Grilli, C.; et al. Survival and toxicities after (90)y transarterial radioembolization of metastatic colorectal cancer in the resin registry. Radiology 2022, 305, 228–236. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Alexander, L.; Anderson, H.R.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Burnett, R.; Casey, D.; Coates, M.M.; Cohen, A.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.; Conte, C.; Tumino, E.; Parisi, G.; Marceglia, S.; Metrangolo, S.; Eggenhoffner, R.; Bresci, G.; Cabibbo, G.; Giacomelli, L. Transarterial radioembolization for hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- van der Geest, L.G.; Lam-Boer, J.; Koopman, M.; Verhoef, C.; Elferink, M.A.; de Wilt, J.H. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin. Exp. Metastasis 2015, 32, 457–465. [Google Scholar] [CrossRef]
- Mulcahy, M.F.; Mahvash, A.; Pracht, M.; Montazeri, A.H.; Bandula, S.; Martin, R.C.G., 2nd; Herrmann, K.; Brown, E.; Zuckerman, D.; Wilson, G.; et al. Radioembolization with chemotherapy for colorectal liver metastases: A randomized, open-label, international, multicenter, phase iii trial. J. Clin. Oncol. 2021, 39, 3897–3907. [Google Scholar] [CrossRef]
- Boas, F.E.; Brody, L.A.; Erinjeri, J.P.; Yarmohammadi, H.; Shady, W.; Kishore, S.; Sofocleous, C.T. Quantitative measurements of enhancement on preprocedure triphasic ct can predict response of colorectal liver metastases to radioembolization. AJR Am. J. Roentgenol. 2016, 207, 671–675. [Google Scholar] [CrossRef]
- Boas, F.E.; Kamaya, A.; Do, B.; Desser, T.S.; Beaulieu, C.F.; Vasanawala, S.S.; Hwang, G.L.; Sze, D.Y. Classification of hypervascular liver lesions based on hepatic artery and portal vein blood supply coefficients calculated from triphasic ct scans. J. Digit. Imaging 2015, 28, 213–223. [Google Scholar] [CrossRef]
- Boas, F.E.; Bodei, L.; Sofocleous, C.T. Radioembolization of colorectal liver metastases: Indications, technique, and outcomes. J. Nucl. Med. 2017, 58, 104s–111s. [Google Scholar] [CrossRef]
- Sangro, B.; Iñarrairaegui, M.; Bilbao, J.I. Radioembolization for hepatocellular carcinoma. J. Hepatol. 2012, 56, 464–473. [Google Scholar] [CrossRef]
- Chao, Y.; Chung, Y.H.; Han, G.; Yoon, J.H.; Yang, J.; Wang, J.; Shao, G.L.; Kim, B.I.; Lee, T.Y. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in asian patients with hepatocellular carcinoma: Final results of the start trial. Int. J. Cancer 2015, 136, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, J.F.; Chapiro, J. Sorafenib in combination with transarterial chemoembolization for the treatment of hepatocellular carcinoma. Clin. Adv. Hematol. Oncol. H&O 2016, 14, 585–587. [Google Scholar]
- Geschwind, J.F.; Kudo, M.; Marrero, J.A.; Venook, A.P.; Chen, X.P.; Bronowicki, J.P.; Dagher, L.; Furuse, J.; Ladrón de Guevara, L.; Papandreou, C.; et al. Tace treatment in patients with sorafenib-treated unresectable hepatocellular carcinoma in clinical practice: Final analysis of gideon. Radiology 2016, 279, 630–640. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M.; Han, G.; Tak, W.Y.; Yang, J.; Guglielmi, A.; Paik, S.W.; Reig, M.; Kim, D.Y.; Chau, G.Y.; et al. Sorafenib or placebo plus tace with doxorubicin-eluting beads for intermediate stage hcc: The space trial. J. Hepatol. 2016, 64, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Koh, Y.H.; Kim, H.B.; Kim, H.Y.; An, S.; Choi, J.I.; Woo, S.M.; Nam, B.H. Phase ii study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J. Hepatol. 2012, 56, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Reyes, D.K.; Cosgrove, D.; Kamel, I.R.; Bhagat, N.; Geschwind, J.F. Phase ii trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J. Clin. Oncol. 2011, 29, 3960–3967. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Paolillo, R.; Tartaglia, N.; Ramai, D.; Mohan, B.P.; Cotsoglou, C.; Chandan, S.; Ambrosi, A.; Bargellini, I.; Renzulli, M.; et al. Efficacy of combined transarterial radioembolization and sorafenib in the treatment of hepatocarcinoma: A meta-analysis. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2022, 54, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Padia, S.A.; Lam, M.; Chiesa, C.; Haste, P.; Sangro, B.; Toskich, B.; Fowers, K.; Herman, J.M.; Kappadath, S.C.; et al. Clinical, dosimetric, and reporting considerations for y-90 glass microspheres in hepatocellular carcinoma: Updated 2022 recommendations from an international multidisciplinary working group. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yue, X.; Zhang, L.; Liu, C.; Wang, J.; Zhang, H.; Cai, P. Imaging evaluation following transarterial radioembolization with yttrium-90 microspheres downstaging hepatocellular carcinoma: The first case in china. Quant. Imaging Med. Surg. 2023, 13, 2744–2750. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liang, J.; Qu, Z.; Yang, F.; Liao, Z.; Gou, H. Transarterial strategies for the treatment of unresectable hepatocellular carcinoma: A systematic review. PLoS ONE 2020, 15, e0227475. [Google Scholar]
- Li, X.; Wang, Y.; Ye, X.; Liang, P. Locoregional combined with systemic therapies for advanced hepatocellular carcinoma: An inevitable trend of rapid development. Front. Mol. Biosci. 2021, 8, 635243. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. Esmo consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Jeyarajah, D.R.; Doyle, M.B.M.; Espat, N.J.; Hansen, P.D.; Iannitti, D.A.; Kim, J.; Thambi-Pillai, T.; Visser, B.C. Role of yttrium-90 selective internal radiation therapy in the treatment of liver-dominant metastatic colorectal cancer: An evidence-based expert consensus algorithm. J. Gastrointest. Oncol. 2020, 11, 443–460. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Kassab, I.; Massani, M.; Townsend, W.; Singal, A.G.; Soydal, C.; Moreno-Luna, L.; Roberts, L.R.; Chen, V.L.; Parikh, N.D. Tace versus tare for patients with hepatocellular carcinoma: Overall and individual patient level meta analysis. Cancer Med. 2023, 12, 2590–2599. [Google Scholar] [CrossRef]
- Filippi, L.; Bagni, O.; Notarianni, E.; Saltarelli, A.; Ambrogi, C.; Schillaci, O. Pet/ct with (18)f-choline or (18)f-fdg in hepatocellular carcinoma submitted to (90)y-tare: A real-world study. Biomedicines 2022, 10, 2996. [Google Scholar] [CrossRef]
- Lim, T.S.; Rhee, H.; Kim, G.M.; Kim, S.U.; Kim, B.K.; Park, J.Y.; Ahn, S.H.; Han, K.H.; Choi, J.Y.; Kim, D.Y. Alpha-fetoprotein, des-gamma-carboxy prothrombin, and modified recist response as predictors of survival after transarterial radioembolization for hepatocellular carcinoma. J. Vasc. Interv. Radiol. JVIR 2019, 30, 1194–1200.e1191. [Google Scholar] [CrossRef]
- Tohme, S.; Bou Samra, P.; Kaltenmeier, C.; Chidi, A.P.; Varley, P.R.; Tsung, A. Radioembolization for hepatocellular carcinoma: A nationwide 10-year experience. J. Vasc. Interv. Radiol. JVIR 2018, 29, 912–919.e912. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Heller, M.; Lokken, R.P.; Fidelman, N.; Lam, A. Socioeconomic and survival analysis of radioembolization in patients with intrahepatic cholangiocarcinoma: A propensity score-adjusted study. J. Vasc. Interv. Radiol. JVIR 2023, 34, 815–823.e811. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.A.; Wongjarupong, N.; Hassan, M.A.; Taha, W.; Abdalla, A.; Bampoh, S.; Onyirioha, K.; Nelson, M.; Glubranson, L.A.; Wiseman, G.A.; et al. The efficacy, safety, and predictors of outcomes of transarterial radioembolization for hepatocellular carcinoma: A retrospective study. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Kolligs, F.; Arnold, D.; Golfieri, R.; Pech, M.; Peynircioglu, B.; Pfammatter, T.; Ronot, M.; Sangro, B.; Schaefer, N.; Maleux, G.; et al. Factors impacting survival after transarterial radioembolization in patients with hepatocellular carcinoma: Results from the prospective cirt study. JHEP Rep. Innov. Hepatol. 2023, 5, 100633. [Google Scholar] [CrossRef] [PubMed]
- Hermann, A.L.; Dieudonné, A.; Ronot, M.; Sanchez, M.; Pereira, H.; Chatellier, G.; Garin, E.; Castera, L.; Lebtahi, R.; Vilgrain, V. Relationship of tumor radiation-absorbed dose to survival and response in hepatocellular carcinoma treated with transarterial radioembolization with (90)y in the sarah study. Radiology 2020, 296, 673–684. [Google Scholar] [CrossRef]
- Stella, M.; Braat, A.J.A.T.; van Rooij, R.; de Jong, H.W.A.M.; Lam, M.G.E.H. Holmium-166 radioembolization: Current status and future prospective. CardioVascular Interv. Radiol. 2022, 45, 1634–1645. [Google Scholar] [CrossRef]
- Avanzo, M.; Wei, L.; Stancanello, J.; Vallières, M.; Rao, A.; Morin, O.; Mattonen, S.A.; El Naqa, I. Machine and deep learning methods for radiomics. Med. Phys. 2020, 47, e185–e202. [Google Scholar] [CrossRef]




| Whole Cohort | HCC | CRC Liver Metastases | p Significance | |
|---|---|---|---|---|
| Number of patients | 39 | 23 | 16 | NA |
| Mean age (SD), years | 63.59 ± 15.66 | 66.77 ± 13.67 | 58.60 ± 17.46 | ns |
| Sex (m) | 27 | 17 (73.9%) | 10 (62.5%) | ns |
| ECOG performance status 1 | 7 | 4 | 3 | ns |
| Palliative intent of TARE (n. patients) | 4 | 0 | 4 | ns |
| Patients receiving additional treatments | 18 (46.1%) | 8 (34.7%) | 10 (62.5%) | ns |
| Mean number of liver metastatic sites | 2.1 ± 0.6 | 2.17 ± 0.8 | 2 | ns |
| Bilobar involvement (n. patients) | 12 | 4 (17.39%) | 8 (50%) | ns |
| Mean diameter of the main tumor lesion | 54.32 | 56.82 | 49.75 | ns |
| Mean lobar volume (cm3) | 997.55 | 942.74 | 1067 | ns |
| Mean organ volume (cm3) | 1650.75 | 1648.33 | 1653.67 | ns |
| Mean tumor volume (cm3) | 339.13 | 360.64 | 305.67 | ns |
| Mean injected activity (GBq) | 2.27 | 1.98 ± 0.75 | 2.68 ± 1.01 | ns |
| Mean dose to the tumor (Gy) | 257.56 | 246.93 | 274.11 | ns |
| Mean follow-up (SD), months | 69 (39–91) | 69.07 ± 12.07 | 74.05 ± 6.95 | ns |
| Side effects (n. patients) | 23 | 15 (65.21%) | 9 (56.25%) | ns |
| Radiation-induced side effects (n. patients) | 3 | 3 (13%) | 0 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quartuccio, N.; Ialuna, S.; Scalisi, D.; D’Amato, F.; Barcellona, M.R.; Bavetta, M.G.; Fusco, G.; Bronte, E.; Musso, E.; Bronte, F.; et al. The Influence of Additional Treatments on the Survival of Patients Undergoing Transarterial Radioembolization (TARE). Curr. Oncol. 2024, 31, 1504-1514. https://doi.org/10.3390/curroncol31030114
Quartuccio N, Ialuna S, Scalisi D, D’Amato F, Barcellona MR, Bavetta MG, Fusco G, Bronte E, Musso E, Bronte F, et al. The Influence of Additional Treatments on the Survival of Patients Undergoing Transarterial Radioembolization (TARE). Current Oncology. 2024; 31(3):1504-1514. https://doi.org/10.3390/curroncol31030114
Chicago/Turabian StyleQuartuccio, Natale, Salvatore Ialuna, Daniele Scalisi, Fabio D’Amato, Maria Rosa Barcellona, Maria Grazia Bavetta, Giorgio Fusco, Enrico Bronte, Emma Musso, Fabrizio Bronte, and et al. 2024. "The Influence of Additional Treatments on the Survival of Patients Undergoing Transarterial Radioembolization (TARE)" Current Oncology 31, no. 3: 1504-1514. https://doi.org/10.3390/curroncol31030114